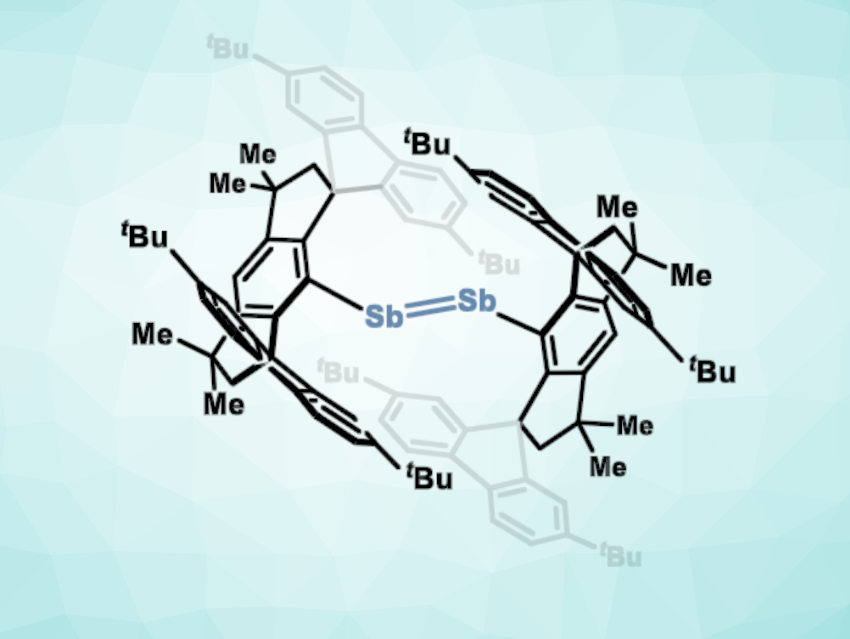
The activation of small molecules using transition metals is an interesting research target. There are some main-group compounds that can show similar reactivity in this context. However, in contrast to group 13 and 14 systems, the transition-metal-like reactivity of low-valent group 15 compounds is less well-studied.
Josep Cornella, Max Planck Institute for Coal Research, Mülheim an der Ruhr, Germany, and colleagues have synthesized a sterically distorted distibene (pictured) and found that it can show transition-metal-like reactivity towards H2 and ethylene. The team reacted a dibromo-funktionalized Sb precursor with Cp*2Co in tetrahydrofuan (THF) or toluene at ambient temperature under argon to obtain the distibene. The product has a distorted, dimeric structure.
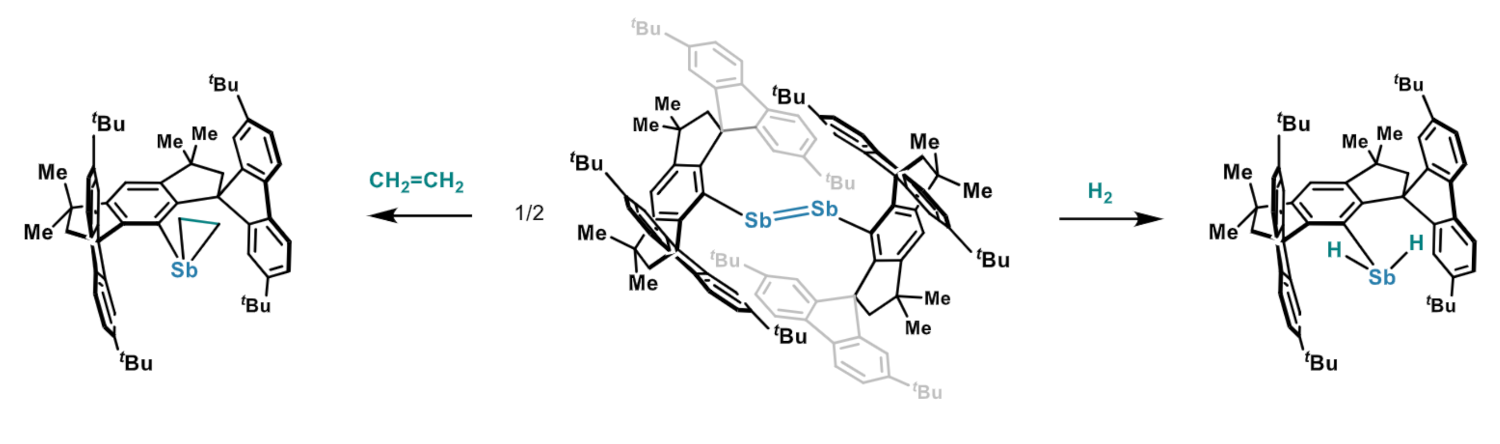
The researchers then tested the reactivity of the distibene towards H2 and ethylene (example reactions pictured). They found that under 1.2 bar of H2 or ethylene at 60 °C, the distibene was converted into the corresponding antimony dihydride or stibacyclopropane, respectively. The team proposes that the reactions involve the in-situ generation of the monomeric stibinidene. Overall, the work adds a transition-metal-like reactivity of group 15 species to the known activation of H2 and unactivated olefins by group 13 and 14 systems
- Dihydrogen and Ethylene Activation by a Sterically Distorted Distibene,
Yue Pang, Markus Leutzsch, Nils Nöthling, Josep Cornella,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202302071