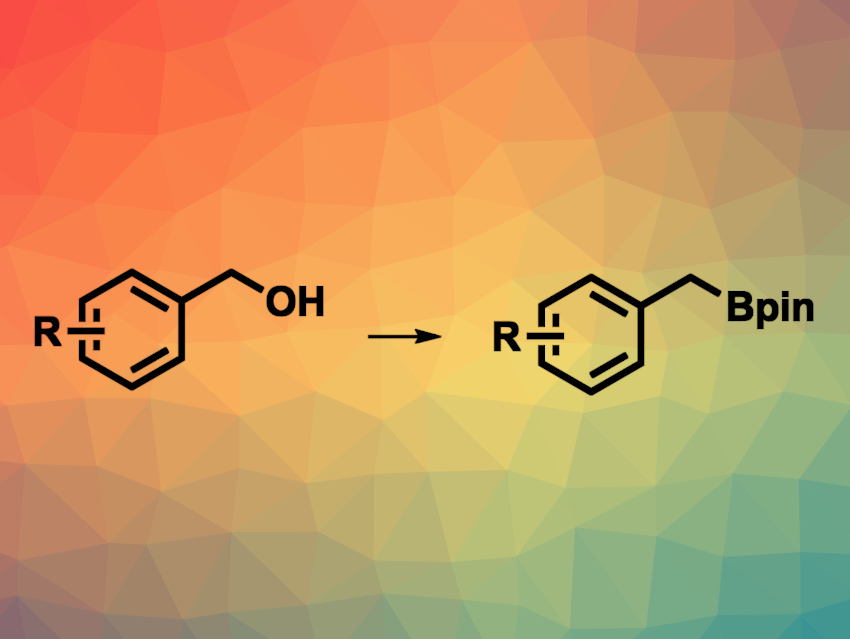
Alcohols are readily available feedstocks for organic synthesis. Cross-coupling reactions using alcohol derivatives are interesting research targets, but direct deoxygenative transformations using unprotected alcohols are challenging. Organoboron compounds are also very useful building blocks. Converting benzylic alcohols to benzylic boronate esters, for example, could be a promising way to obtain useful reagents.
Hua Zhang, South-Central Minzu University, Wuhan, China, and Hangzhou Normal University, China, and colleagues have developed a method for the transition-metal-free, iodine-catalyzed direct borylation of benzylic alcohols (general reaction pictured). The team reacted a variety of benzylic alcohols with bis(catecholato)diboron (B2cat2) in the presence of iodine and 1-methyl-2-pyrrolidone (NMP) at 120 °C. This was followed by the addition of pinacol in the presence of Et3N at room temperature to obtain the desired borylated products.
The products were obtained in moderate to high yields. Both electron-donating and electron-withdrawing substituents were tolerated—including halogen substituents, which could allow for additional transformations of the products. The team proposes that benzylic iodide and radical intermediates are likely involved in the reaction.
- Iodine-Catalyzed Borylation of Benzylic Alcohols,
Chunyu Yin, Lu Luo, Hua Zhang,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c00367