In nature, there are diverse S-adenosylmethionine (SAM)-dependent methyltransferase (MT) enzymes that can catalyze highly selective methylation reactions across a wide spectrum of substrates, including metabolites and macromolecules. Such methylation reactions can be useful in synthetic organic chemistry, for example, for late-stage methylations of bioactive compounds in drug discovery. Beyond methylation reactions, MTs have also been used to transfer other alkyl groups using analogs of SAM, but access to such analogs has not been straightforward so far.
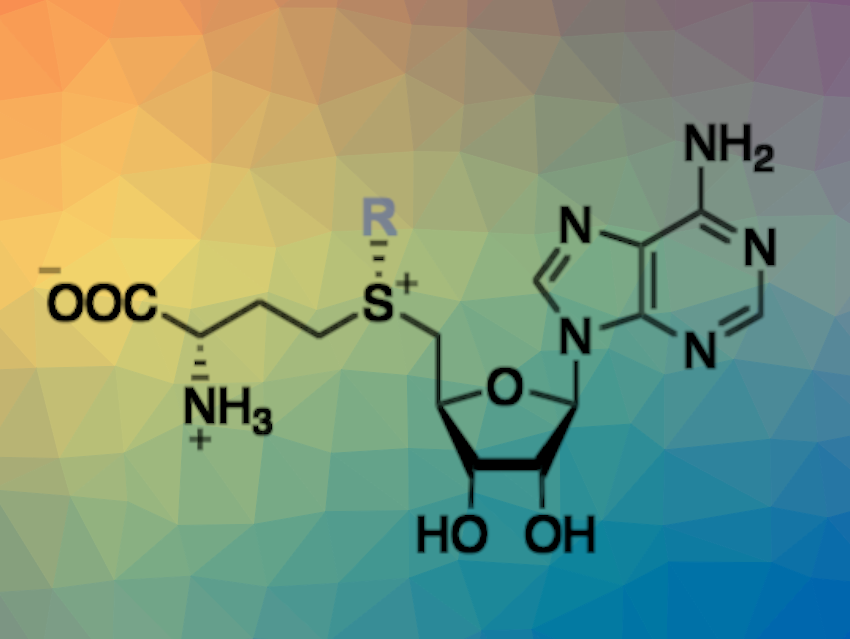
Stephan C. Hammer, Bielefeld University, Germany, and colleagues have engineered transferases for the synthesis of SAM analogs using readily available iodoalkanes via an enzymatic alkylation of S-adenosyl-L-homocysteine (reaction pictured below). The team optimized the enzymes by mutating multiple hydrophobic amino acids in structurally dynamic active-site regions, which were identified using molecular dynamics (MD) simulations.
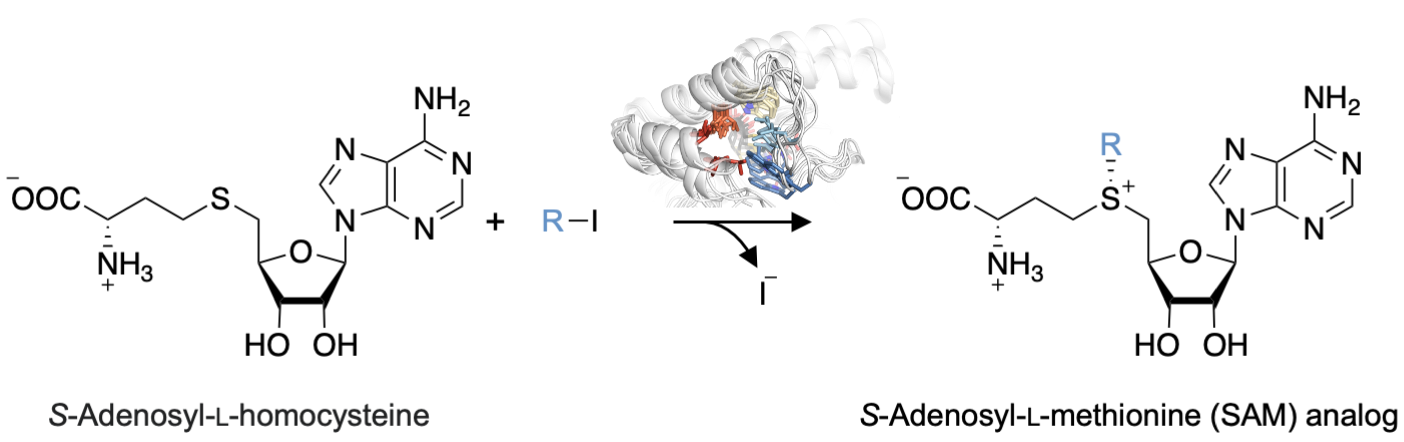
The product scope of the optimized transferases was extended to include SAM analogs with, for example, cyclopropyl or aromatic moieties. Overall, the work could be useful in building a platform for a selective biocatalytic alkylation chemistry that combines the broad range of commercially available haloalkanes with the selectivity and activity of enzymes.
- Efficient Transferase Engineering for SAM Analog Synthesis from Iodoalkanes,
Kai H. Schülke, Jana S. Fröse, Alina Klein, Marc Garcia-Borràs, Stephan C. Hammer,
ChemBioChem 2024.
https://doi.org/10.1002/cbic.202400079