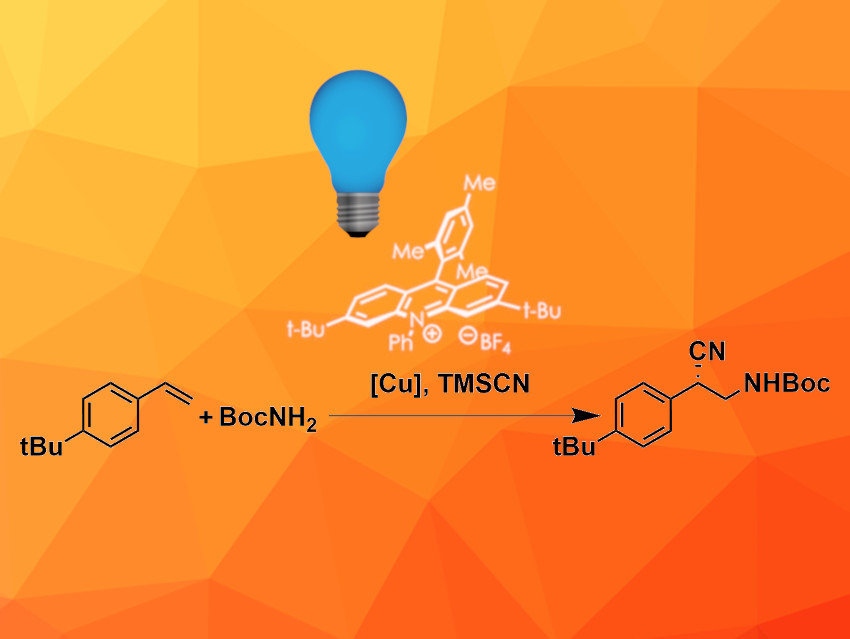
Chiral β-amino nitriles are found, e.g., in natural products, agrochemicals, and pharmaceuticals. They can also serve as useful synthetic precursors. Established enantioselective synthesis routes for β-amino nitriles often have limited substrate scopes due to side reactions or require difficult-to-synthesize starting materials. However, these challenges can be overcome using tailored catalyst systems that provide high enantioselectivity and suppress side reactions.
David A. Nicewicz, University of North Carolina at Chapel Hill, USA, and colleagues have developed a method for the enantioselective aminocyanation and oxycyanation of alkenes. They combined an acridinium-based organic photoredox catalyst (pictured in white) and a Cu(II) catalyst with an isopropyl cyclopropane serinate-derived bisoxazoline ligand. The photoredox catalyst is activated by light and oxidizes the alkene substrate to a cationic radical. This intermediate quickly reacts with a nucleophile, e.g, an amine, to form a radical species which then undergoes copper-catalyzed cyanation to give the final product.
The aminocyanation reaction was performed in acetonitrile at 5 °C under blue LED light. TMSCN was used as the cyanide source, Cu(OTf)2 as a copper precursor, and tert-butyl hydroperoxide as an oxidant. The target products were obtained in moderate yields but with high enantiomeric excess. The reaction is compatible with a wide range of differently functionalized styrene derivatives and different N-nucleophiles.
The method was successfully extended to carboxylic acid nucleophiles, for which the catalytic system proved to be well-suited because it suppresses the undesired decarboxylation of the acid precursors. The target lactones were obtained in moderate to high yields with high enantiomeric excess. Derivatives of ibuprofen and esterone were also successfully functionalized, indicating possible uses of the developed method in pharmaceutical chemistry.
- Enantioselective Amino- and Oxycyanation of Alkenes via Organic Photoredox and Copper Catalysis,
Siran Qian, Tanya M. Lazarus, David A. Nicewicz,
J. Am. Chem. Soc.2023.
https://doi.org/10.1021/jacs.3c06936