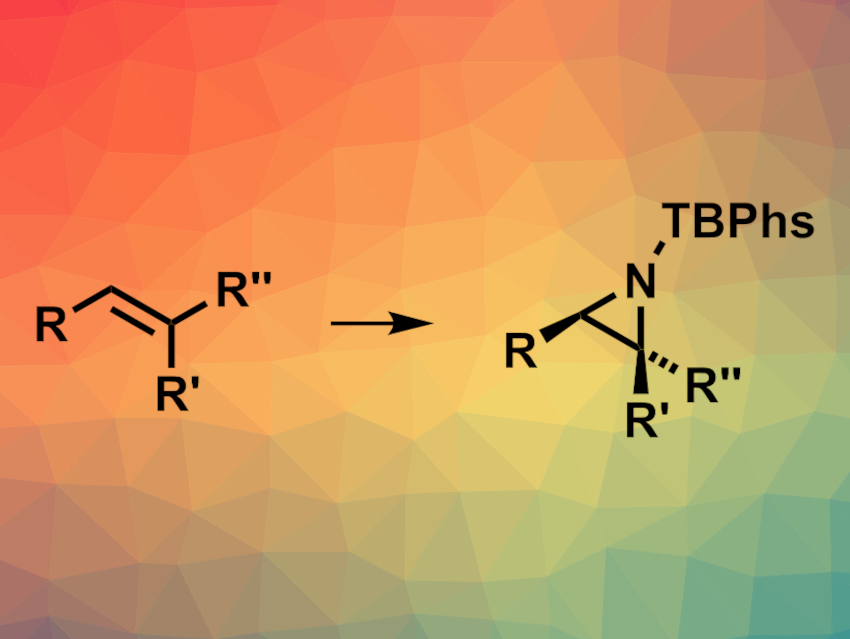
Aziridines are three-membered rings with a single nitrogen atom. They are similar to epoxides and can be useful intermediates in organic synthesis. Methods for the enantioselective synthesis of aziridines are, thus, useful. However, approaches for the asymmetric preparation of trisubstituted aziridine derivatives are rare.
Benjamin Darses, Université Grenoble Alpes, CNRS, Grenoble, France, Marie Sircoglou, Université Paris-Saclay, CNRS, Institut de Chimie Moléculaire et des Matériaux d’Orsay, France, Philippe Dauban, Université Paris-Saclay, CNRS, Institut de Chimie des Substances Naturelles, Gif-sur-Yvette, France, and colleagues have developed a method for the enantioselective intermolecular aziridination of substituted alkenes with sulfamates using a rhodium(II) catalyst.
The team reacted a variety of styrene derivatives and aliphatic alkenes, including trisubstituted alkenes, with p–tBu-phenylsulfamate (TBPhsNH2) using a dirhodium(II) tetracarboxylate (Rh2(S-tfpttl)4, (S)-tfpttl = (S)-3,3-dimethyl-2-(4,5,6,7-tetrafluoro-1,3-dioxoisoindolin-2-yl)butanoic acid) as the catalyst, commercially available PhI(OPiv)2 as the oxidant, and pentafluorobenzoic acid as an additive. The reactions were performed in toluene at –15 °C.
The desired aziridines were obtained in generally high yields with enantiomeric excesses of up to 99 %. The reaction tolerates low catalyst loadings. It has a wide scope, including mono-, di-, and trisubstituted alkenes, and can be used for the late-stage functionalization of complex molecules. The team also performed an aziridination reaction on a gram scale with an isolated yield of 85 %.
- Rhodium(II)-Catalyzed Enantioselective Intermolecular Aziridination of Alkenes,
Vincent Boquet, Ali Nasrallah, Alejandro L. Dana, Erwan Brunard, Pablo H. Di Chenna, Fernando J. Duran, Pascal Retailleau, Benjamin Darses, Marie Sircoglou, Philippe Dauban,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.2c07337