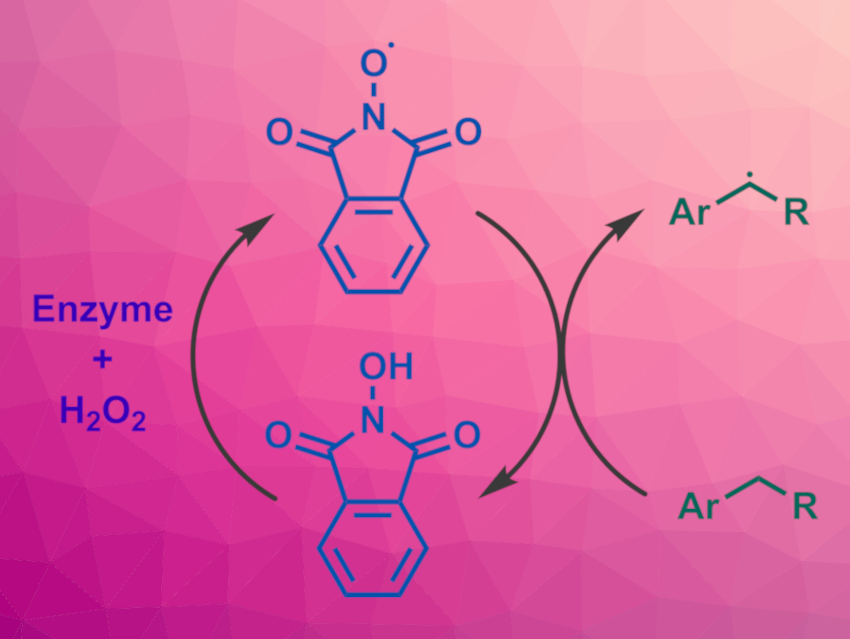
The selective functionalization of alkylbenzenes is useful in organic synthesis, e.g., for the preparation of pharmaceutically active compounds. Redox mediators such as N-hydroxy compounds can be used in this context to activate C–H bonds via hydrogen atom transfer (HAT) reactions. An oxidant is used to generate an N-oxyl radical from the redox mediator, which abstracts a hydrogen atom from the substrate and generates a reactive alkyl radical. This radical can then be functionalized.
Jeffrey D. Martell, University of Wisconsin−Madison, USA, and colleagues have developed a system for the functionalization of alkylbenzenes under mild conditions that combines an enzyme with a redox mediator. The team used N-hydroxyphthalimide (NHPI) as the redox mediator (pictured in the center), H2O2 as an oxidant, and horseradish peroxidase (HRP) as the enzyme.
Using this system, different alkylbenzenes can be oxidized to the corresponding ketones or aldehydes in moderate to high yields at room temperature under air. When the team increased the concentrations of the enzyme and NHPI and removed O2, they obtained a benzylic NHPI adduct instead, resulting from a coupling of the radical form of the mediator and the benzylic radical. Both these adducts and the synthesized ketones and aldehydes can be useful substrates for further functionalizations.
- A Horseradish Peroxidase–Mediator System for Benzylic C–H Activation,
Mario A. Cribari, Maxwell J. Unger, Jeffrey D. Martell,
ACS Catal. 2022.
https://doi.org/10.1021/acscatal.2c03424