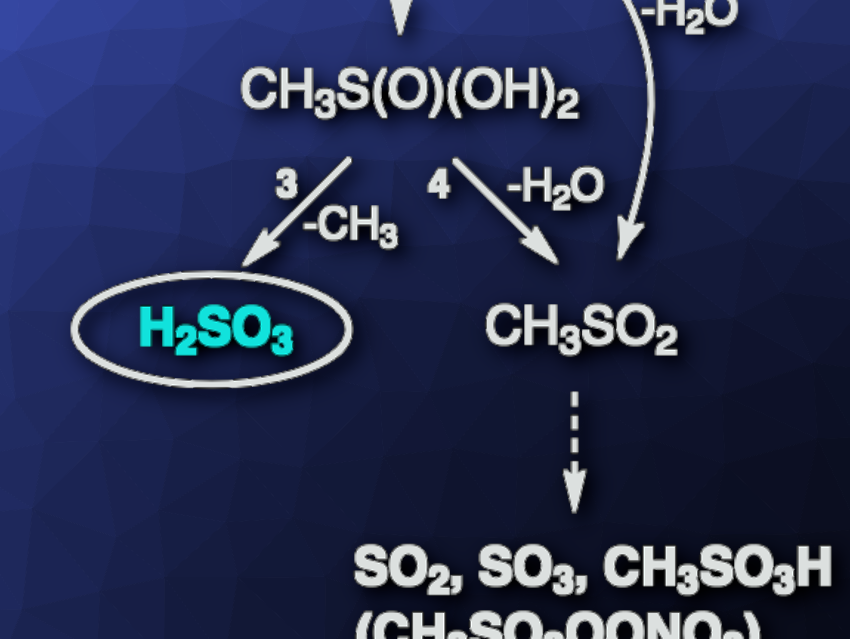
The potential formation of sulfurous acid (H2SO3) via a reaction of SO2 and H2O in an aqueous SO2 solution has been proposed. However, experimental proof of the presence of H2SO3 in such a solution using spectroscopic methods, however, has been elusive so far. H2SO3 has also been discussed as a possible intermediate product in the course of dimethyl sulfide (H3CSCH3) oxidation in the atmosphere. Dimethyl sulfide can first be oxidized to dimethyl sulfoxide (H3CS(O)CH3), which can be further oxidized by OH radicals to methanesulfinic acid (H3CS(O)OH).
Torsten Berndt, Institute for Tropospheric Research (TROPOS), Leipzig, Germany, and colleagues have investigated the gas-phase reaction of OH radicals with H3CS(O)CH3, with a focus on the possible generation of H2SO3 from OH and H3CS(O)OH. The team conducted experiments were conducted in a laminar flow tube with 1 bar of air, an OH radical source such as the photolysis of isopropyl nitrite or the ozonolysis of tetramethylethylene, and different amounts of water vapor. They analyzed the products using mass spectrometry.
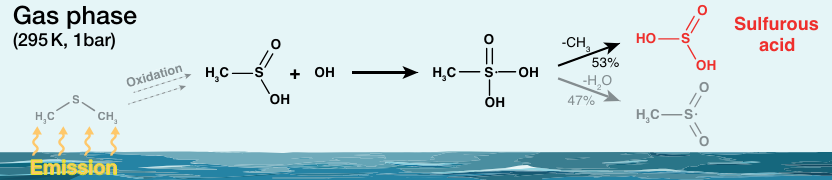
The researchers found that H2SO3 is formed in the OH radical-initiated gas-phase oxidation of methanesulfinic acid. Other main products are SO2, SO3, and methanesulfonic acid. In order to assess the potential role of H2SO3 in the atmosphere, the team calculated its global annual production using a chemistry climate model. They found a predicted annual H2SO3 production of ca. 8 million tons from the OH + H3CS(O)OH reaction. They calculated a lifetime of the species of at least one second at atmospheric humidity. Overall, the work shows experimentally that H2SO3, once formed in the gas phase, is kinetically stable enough to allow its characterization and subsequent reactions.
- Gas‐Phase Formation of Sulfurous Acid (H2SO3) in the Atmosphere,
Torsten Berndt, Erik H. Hoffmann, Andreas Tilgner, Hartmut Herrmann,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202405572