Most often, aromatic rings contain six or more π-electrons, such as in benzene or the cyclopentadienyl anion. In contrast, examples of 2π-aromatics are very rare and remain limited to three- and four-membered rings, unless the species are stabilized in the coordination sphere of heavy metals.
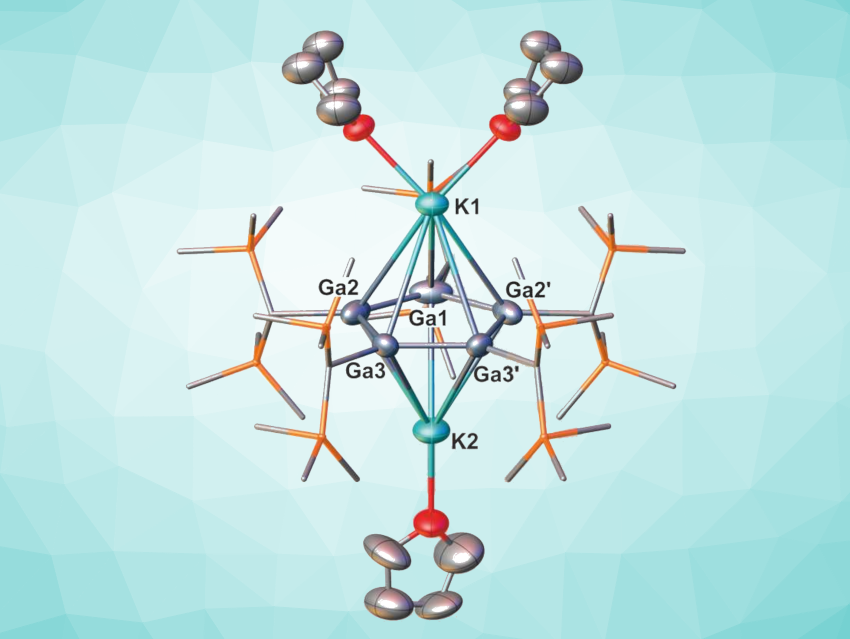
Robert Kretschmer, Chemnitz University of Technology, Germany, and colleagues have synthesized a dipotassium cyclopentagallene (pictured), which features a unique example of a five-membered aromatic ring stabilized by only two π-electrons. The dipotassium cyclopentagallene was obtained via a room-temperature salt metathesis reaction of five equivalents of a gallium(I) β-diketiminate with seven equivalents of potassium bis(trimethylsilyl)methanide. The product was isolated in the form of green crystals.
Single-crystal X-ray diffraction showed a planar Ga5 ring with almost equal gallium–gallium bond lengths. The green color of the complex can be attributed to π–π*-transitions, consistent with an aromatic ring system. The team quantified the ring current computationally, using nucleus-independent chemical shift (NICS) and ring current density calculations. The results substantiate the proposed (weak) aromatic character of the Ga5 ring and suggest that aromatic stabilization can occur in species that had previously been considered below the minimum π-electron count in a five-atom ring fragment.
- A Planar Five‐Membered Aromatic Ring Stabilized by Only Two π‐Electrons,
Oleksandr Kysliak, Simon H. F. Schreiner, Niklas Grabicki, Phil Liebing, Florian Weigend, Oliver Dumele, Robert Kretschmer,
Angew. Chem. Int. Ed. 2022.
https://doi.org/10.1002/anie.202206963