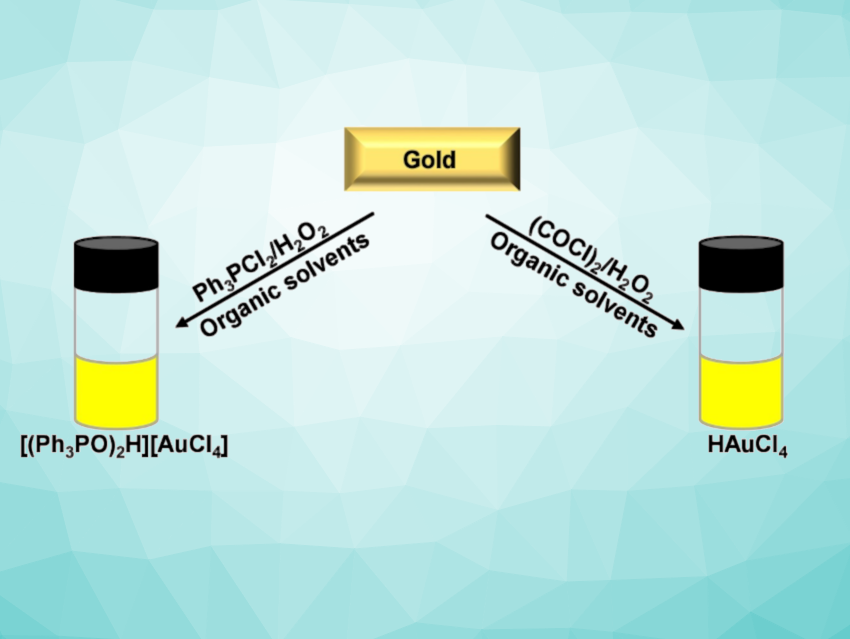
Noble metals such as gold, palladium, platinum, and copper not only have value in jewelry or as a form of investment. They also have broad applications in, e.g., electronics, medicine, or catalysis. The leaching of these metals from their ores or secondary sources such as e-waste is essential for their production and recycling, but the necessary processes are often environmentally damaging. For example, gold is leached industrially using highly toxic cyanide, and the use of mercury in artisanal gold mining has resulted in widespread mercury pollution.
Jason Love and colleagues, University of Edinburgh, UK, have found that noble metals can be dissolved in minutes using mixtures of triphenylphosphine dichloride or oxalyl chloride and hydrogen peroxide in organic solvents (pictured), forming soluble chloridometalate complexes. Almost quantitative dissolution of metallic Au, Pd, and Cu was observed within minutes at room temperature. The dissolution of Pt is slower.
After dissolution, the resulting metal chloride compounds can be separated using known extraction and precipitation techniques. After reduction and recovery of the metal, triphenylphosphine dichloride can be regenerated and reused.
This method can also be used in e-waste recycling, rapidly dissolving gold, copper, and nickel from waste CPUs with subsequent quantitative separation. According to the researchers, this approach to metal dissolution has potential applications in the hydrometallurgical leaching and purification of noble metals from ores, spent catalysts, and electronic and nanomaterial wastes.
- Rapid Dissolution of Noble Metals in Organic Solvents,
Abhijit Nag, Carole Morrison, Jason Love,
ChemSusChem 2022.
https://doi.org/10.1002/cssc.202201285