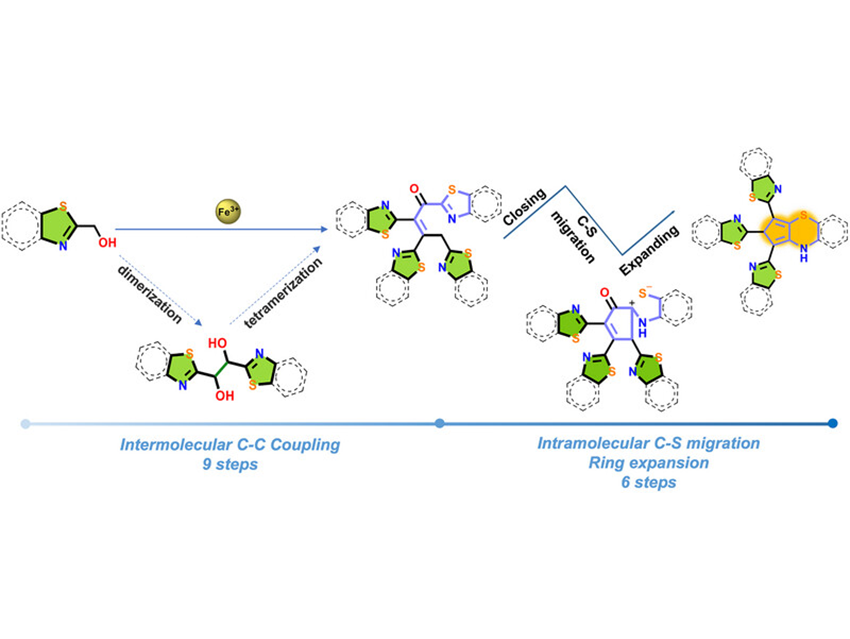
Aggregation reactions typically yield oligomers or polymers through simple, single-step processes. A promising strategy to synthesize oligomers while enriching intermediate steps involves developing ultra-long domino reactions by selecting appropriate substrates and conditions.
Ming-Hua Zeng and colleagues at Hubei University (Wuhan, China) employed a simple metal salt, FeCl3·6H2O, as a promoter to achieve a remarkable 15-step domino reaction sequence. Using the chelating substrate benzothiazole-2-methanol, they synthesized a substituted tricyclic system (2,9-dihydrobenzo[b]cyclopenta[e][1,4]thiazine—the third reported isomer of this tricycle) along with its dehydrogenated derivatives.
Through in-depth investigation using electrospray ionization mass spectrometry (ESI-MS), the team captured key intermediates (dimer, trimer, tetramer). Combined with control experiments and theoretical calculations, they elucidated the reaction pathway: Fe3+ coordination activates the substrate, triggering elimination and triple coupling, followed by thiazole ring expansion via sulfur atom migration, and concluding with dehydration/dehydrogenation to yield the final products. Preliminary photophysical studies indicate these aggregates possess potential fluorescence applications.
This work demonstrates that coordinating chelating substrates with 3d transition metal ions can significantly enrich domino reaction pathways, leading to novel heterocyclic aggregates. Furthermore, it underscores the fundamental importance of integrating techniques like ESI-MS, crystallography, and theoretical calculations for analyzing complex reaction systems—a prerequisite for achieving reaction control and efficiently synthesizing complex aggregates with specific structures and functions.
- Elucidating the Fe(III) Directed 15-Step Domino Inter- and Intramolecular Progressive Coordinative Oligomerization of a Heterocycle Aggregate
Kai-Bin Chen, Ting-Ting Wang, Zhi-Wei Xu, Ning Tian, Jin Cai, Wen-Yu Qiu, Bin Zhang, Zheng Yin, Bin Liu, Ming-Hua Zeng,
Aggregate 2025
https://doi.org/10.1002/agt2.70094