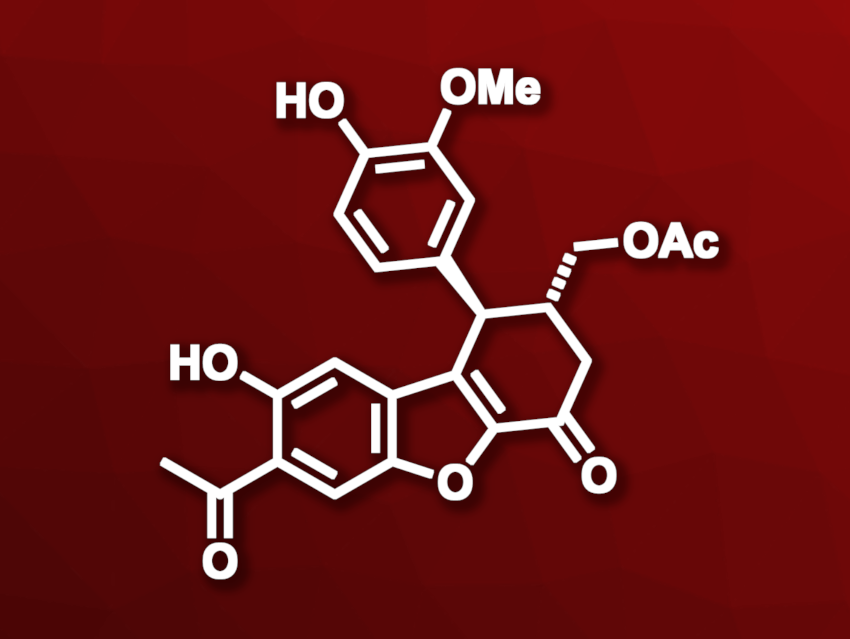
Propolis is a resinous mixture produced by honey bees. It has long been used in traditional medicine, and its constituents can be interesting research subjects. (+)-Propolisbenzofuran B (pictured), for example, can be found in the methanol extract of Brazilian propolis and has shown modest cytotoxic effects. It is an interesting target for synthesis, yet no asymmetric total synthesis of the compound had been reported so far.
Saumen Hajra, Centre of Biomedical Research, Sanjay Gandhi Postgraduate Institute of Medical Sciences (SGPGIMS) Campus, Lucknow, India, and colleagues have performed the first asymmetric total synthesis of (+)-propolisbenzofuran B. The team started from an O-acetyl derivative of 2,5-dibromo-p-hydroquinone, which was reacted with a protected hex-5-yne-ol in a tandem Sonograshira coupling/deacetylative annulation reaction to build the benzofuran substructure of the product, followed by deprotection. The resulting intermediate was deacylated and converted to an O-isopropyl derivative, followed by oxidation to create an acid group.
The carboxylic acid was then coupled with a chiral oxazolidinone, followed by an Evans syn-aldol reaction with O-isopropyl vanillin. A Friedel-Crafts cyclization was then used to close the third fused ring and the chiral auxiliary was removed. Next, the team performed a Pd(PPh3)4-catalyzed Stille coupling with (α-ethoxyvinyl)tributyltin. Final oxidation and deprotection steps then gave the desired (+)-propolisbenzofuran B.
The synthesis was completed in eleven steps and with an overall yield of 11.9 %. The work could allow the synthesis of a library of propolisbenzofuran B derivatives fur further bioactivity studies.
- Asymmetric total synthesis of (+)-propolisbenzofuran B,
Biswajit Sen, Mainak Bera, Mayasankar Singh, Saumen Hajra,
Chem. Commun. 2023.
https://doi.org/10.1039/D3CC02054A




Thanks, it is nice to hear this synthesis.