Chalcogen bonds are noncovalent interactions, similar to hydrogen bonds. In a chalcogen bond, a positively polarized chalcogen atom interacts with an electron-donating Lewis base, i.e., it is a σ–hole interaction. These interactions can be useful, e.g., in recognition and sensing or in crystal engineering and self-assembly. Known chalcogen bonds most often involve Te or Se, and S to a lesser extent. Due to its high electronegativity, oxygen has been considered ill-suited for forming chalcogen bonds, and little is known about oxygen-centered chalcogen bonds.
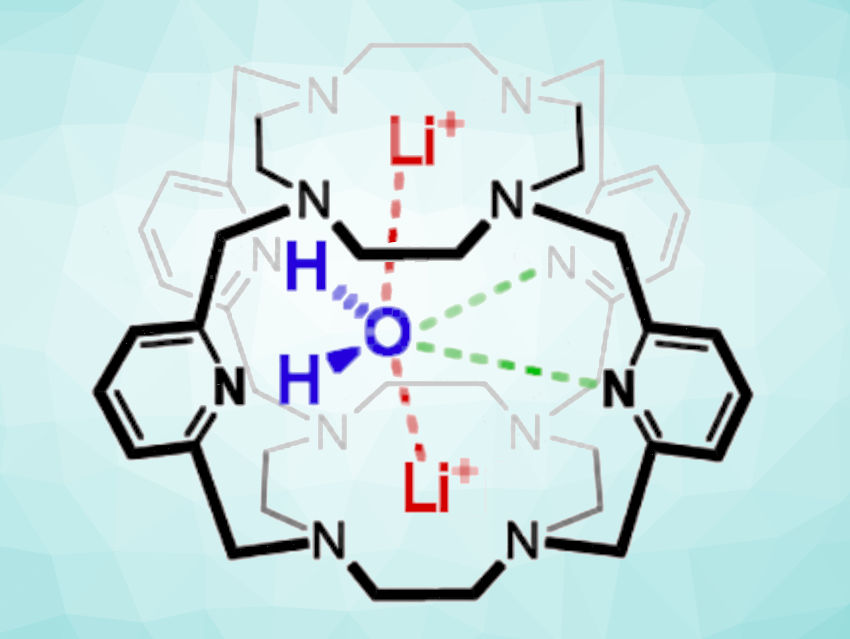
Qing He, Hunan University, Changsha, China, and colleagues have discovered the first-ever chalcogen bonding centered at a water oxygen atom. The team used a molecular cage to stabilize a Li+···H2O···Li+ complex (pictured), in which water is trapped between two lithium ions and strongly polarized. The molecular cage is constructed by connecting two cyclen (1,4,7,10-tetraazacyclododecane) macrocycles using four pyridinyl-containing linkers. The resulting cage features eight amine-type nitrogen atoms in a cube-like arrangement (in the two stacked cyclen rings) and 4 pyridinyl nitrogen atoms along the edges/linkers.
The team grew single crystals of the cage complex and analyzed them using X-ray crystallography. The complex was also characterized using NMR spectroscopy and studied using density functional theory (DFT) calculations. The results confirmed that one water molecule is “clamped” between two lithium ions in the cage. This results in a polarization of the water molecule, indicated by an unusual chemical shift of up to 10 ppm in the 1H NMR spectra.
Two of the pyridine nitrogen atoms have lone electron pairs pointing toward the water’s oxygen atom, and the team found comparatively short distances between those nitrogen atoms and the oxygen atoms, which they attribute to unusual oxygen-centered chalcogen bonding (interaction pictured in green). This was supported by theoretical calculations, which indicate the existence of energetically favorable noncovalent bonds between oxygen and the pyridinyl nitrogen atoms and show that the relevant nitrogen lone pairs can align with the σ-hole of the oxygen. The work, thus, provides evidence of the first water O-centered chalcogen bond.
- The Missing Chalcogen Bonding Donor: Strongly Polarized Oxygen of Water,
Qinpeng Zhang, Ke Luo, Wei Zhou, Aimin Li, Qing He,
J. Am. Chem. Soc. 2024.
https://doi.org/10.1021/jacs.3c13604