While C=C bonds are very common, double bonds between heavier main-group elements are much rarer. Sterically demanding substituents can be used to stabilize these unusual species, which has led to the isolation of disilenes, digermenes, distannenes, and diplumbenes. In contrast to alkenes, these compounds generally have trans-bent structures in which the four substituents are not arranged in one plane. Similar to these ditetrylenes, homo- and heterodiatomic double bonds are known between heavier group 15 and group 13 elements.
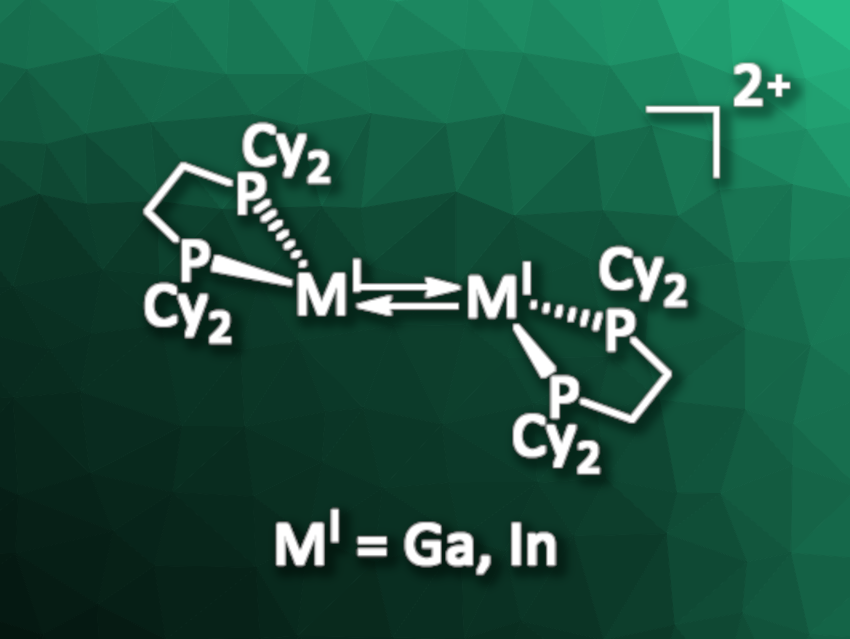
Ingo Krossing, University of Freiburg, Germany, and colleagues have synthesized the first dicationic trans-bent digallene and diindene compounds. The team reacted [M(PhF)2][pf] (M = Ga, In; [pf]– = [Al(ORF)4]–; RF = C(CF3)3) with bisdicyclohexylphosphinoethane (dcpe) in oDFB (ortho-difluorobenzene) in a 1:1 ratio. The solutions were layered with n-pentane, and the researchers obtained [{Ga(dcpe)}2][pf]2 and [{In(dcpe)}2][pf]2 (general structure of the cation pictured) in good yields (77 % and 86 %, respectively) as red crystals.
The products, which are ditrielenes, were characterized using single-crystal X-ray diffraction and powder X-ray diffraction, as well as NMR, Raman, and IR spectroscopy. The team found that the compounds have trans-bent structures with surprisingly short Ga–Ga and In–In bonds. The researchers also performed quantum-chemical calculations to investigate the bonding situation in the ditrielenes and confirmed that the compounds have a non-classical double bond character. Reactivity studies showed that [{Ga(dcpe)}2]2+ readily reacts with 1-hexene, cyclooctyne, diphenyldisulfide, and diphenylphosphine in solution, undergoing, e.g., [2+2] cycloaddition reactions or oxidative additions.
- Structures, Bonding Analyses and Reactivity of a Dicationic Digallene and Diindene Mimicking trans‐bent Ditetrylenes,
Antoine Barthélemy, Harald Scherer, Michael Daub, Alexis Bugnet, Ingo Krossing,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202311648