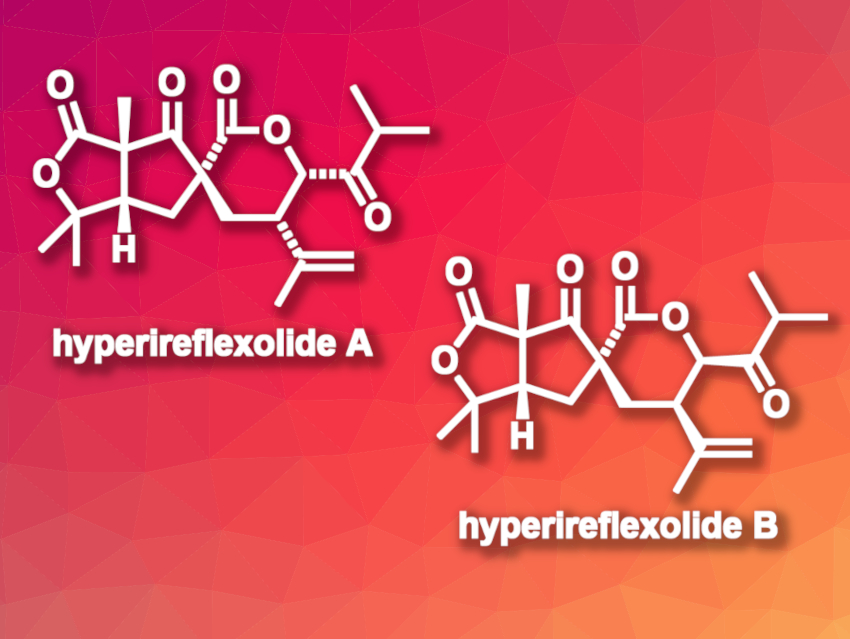
Hyperireflexolides A and B (pictured) are natural products that were first isolated from the plant Hypericum reflexum. It has been proposed that the two compounds could be highly rearranged polycyclic polyprenylated acylphloroglucinol (PPAP) natural products, a family of compounds also found in Hypericum plants.
Jonathan H. George, The University of Adelaide, Australia, and colleagues have validated this hypothesis by performing the first total syntheses of hyperireflexolides A and B from a simple acylphloroglucinol via dearomatization and fragmentation of the starting material. The team first performed a C-prenylation of the starting acylphloroglucinol, followed by a methylation to give an intermediate containing all carbon atoms of the desired products. A one-electron oxidation then gave a radical intermediate that underwent a 5-exo-trig cyclization, trapping with triplet oxygen, and an in-situ reduction to give enaimeones A and B. The team revised the previously proposed relative configurations of enaimeones A and B.
The obtained enaimeone A was subjected to a retro-Dieckmann fragmentation, followed by an oxidation of with Dess–Martin periodinane to give spirocyclic intermediates, which were converted to hyperireflexolides A and B via thermal α-hydroxy-β-diketone rearrangements. The team also revised the structure of hyperireflexolide B. In addition to this bioinspired synthesis pathway, the team also performed more practical stepwise syntheses of hyperireflexolides A and B via a diastereoselective functionalization of 2-cyclopentenone.
- Bioinspired Total Synthesis of Hyperireflexolides A and B,
Andreas B. zur Bonsen, Christopher J. Sumby, Jonathan H. George,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c02232


Do we know the mechanism of oxidation?
Really lovely chemistry.
Oxidation is very useful.
Structures are remarkably complex.
Super work!
Hi Christopher,
Thank you, I am glad to hear you like our work. The mechanism for the oxidation of the diketone to a triketone likely proceeds through a enolonium species (an enol bound to a hypervalent iodine though a O-I bond) which is then attacked by a molecule of DMP where one acetate is replaced by water. This mechanism was first proposed by Stuart Schreiber. The triketone undergoes then rapid carbonyl-ene reaction. Feel free to contact me directly if you have any further questions.
Andreas