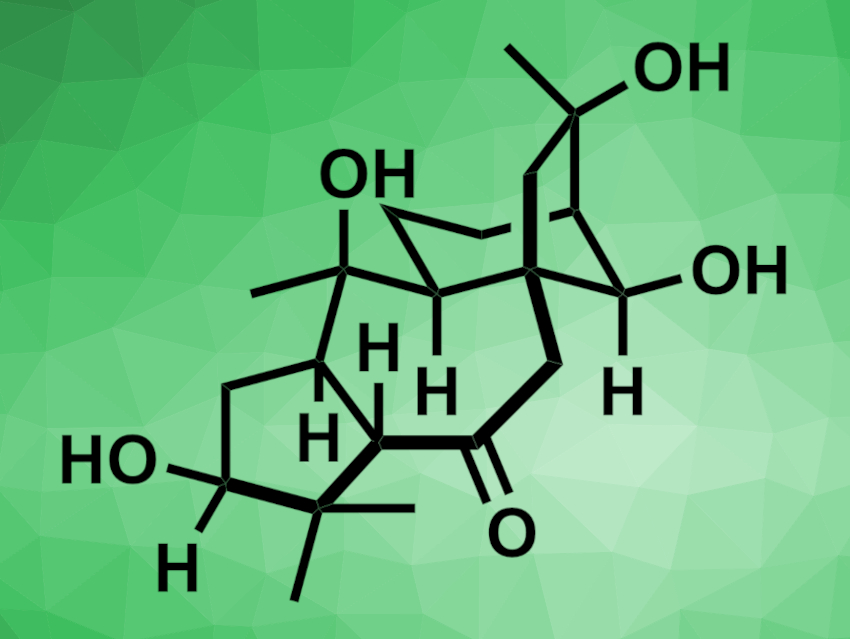
Principinol C (pictured) is a natural product that was isolated from the evergreen shrub Rhododendron principis. It has a complex structure with a 5/7/6/5 tetracyclic core and eight contiguous stereocenters. This makes the compound challenging to prepare, and so far, no total synthesis of principinol C had been reported.
Yanxing Jia, Peking University, Beijing, China, and colleagues have performed the first total synthesis of (−)-principinol C. The first prepared an enyne precursor from a bicyclo[3.2.1]octane derivative. Then, in a key step, an intramolecular Pauson–Khand reaction using Co2(CO)8 at 40–60 °C was employed to build the 7/5-bicyclic ring system of the target product. The desired tetracycle was obtained in 45 % yield. This transformation was followed by a methylation step and subsequent reduction/oxidation and protection/deprotection reactions to obtain the desired (−)-principinol C.
Overall, the team synthesized (−)-principinol C in 16 steps and 2.8 % overall yield starting from a bicyclo[3.2.1]octane ring system. Including the synthesis of the known bicyclo[3.2.1]octane ring system starting from a 2-cyclohexenone, the preparation took 21 steps and gave 0.6 % overall yield. According to the researchers, the work includes is the first application of the intramolecular Pauson–Khand reaction of an enyne to construct a 7,5-bicyclic ring system in natural product synthesis.
- Total Synthesis of (−)-Principinol C,
Tianhao Ma, Hao Cheng, Mallesham Pitchakuntla, Weihao Ma, Yanxing Jia,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.2c08694