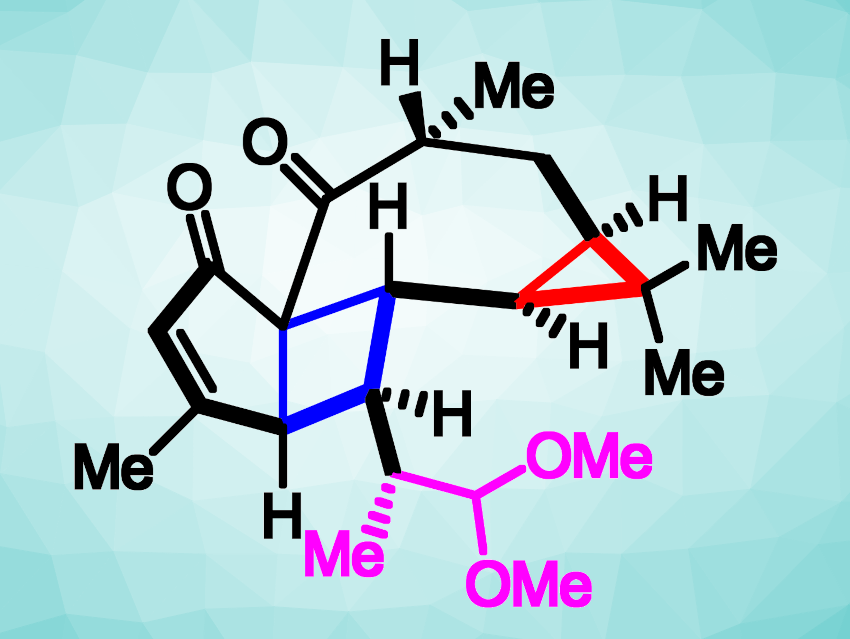
Euphorbia plants, or spurges, contain a wide variety of diterpenes. Many of these natural products have shown useful biological activities. They can be interesting targets for total synthesis. The pepluanol family of natural products, for example, demonstrated immunosuppressive effects, which could be useful to treat autoimmune diseases. Pepluacetal (pictured), a member of this compound family, has a 5-4-7-3 tetracyclic structure with eight stereocenters and is a challenging target for organic synthesis.
Huilin Li, Xuegong She, Lanzhou University, China, and colleagues have performed the first total synthesis of pepluacetal. The team started from a bicyclic diol, which was functionalized using a Mukaiyama aldol reaction and converted to a hydroxyl diazoketone. A photo-induced Wolff rearrangement/lactonization cascade then gave a γ-lactone intermediate. A lactone semi-reduction and a Wittig olefination were then used to obtain a diene, followed by a ring-closing metathesis reaction to form the seven-membered ring. The remaining double bond was then used to introduce the gem-dimethyl cyclopropane unit.
The resulting tetracyclic intermediate was oxidized to introduce a cyclopentanone functionality, which was further transformed to give a diazo ketone-functionalized side chain. The diazo group was then used in a Rh-catalyzed transformation to create a metallo-carbenoid species, which underwent a transannular C(sp3)–H insertion and formed a cyclopentanone bridge. A Baeyer-Villiger oxidation was used to convert the cyclopentanone to a δ-lactone, which was opened to form the side chain of the target product.
Further functional group interconversions and isomerizations ultimately gave pepluacetal. The NMR spectra of the synthetic product were in good agreement with the isolated natural product. The synthesis has 27 linear steps from a known functionalized bicyclic intermediate. The team obtained 20 mg of (+)-pepluacetal in one batch.
- Total Synthesis of the Euphorbia Diterpenoid Pepluacetal,
Meng Liu, Chuanhua Wu, Xingang Xie, Huilin Li, Xuegong She,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202400943


![A Path to Substituted Bicyclo[2.1.1]hexanones](https://www.chemistryviews.org/wp-content/uploads/2024/10/1substitutedbicyclo211hexan2ones_2024-125x94.png)
