Flexible aqueous batteries, such as those used in portable electronics, often contain a hydrogel electrolyte containing water and salt. Using a chemical modification inspired by Nature, Zheng Chen, Jingwen Zhao, Qingdao Institute of Bioenergy and Bioprocess Technology, Chinese Academy of Sciences, and Shandong Energy Institute, Qingdao, China, Guanglei Cui, Qingdao Institute of Bioenergy and Bioprocess Technology and University of the Chinese Academy of Sciences, Beijing, and colleagues have significantly increased the salt stability of hydrogels used in sodium-ion batteries. A simple methylation of the hydrogel’s structural polymer prevented salting-out and improved battery capacity and cycling performance.
Hydrogel Electrolytes
Sodium-ion batteries constitute a promising alternative to lithium-ion batteries, since they contain cheaper and more eco-friendly materials than lithium-ion batteries. However, new batteries require the development of many new components, all of which have to be adapted to the sodium ion. One of the most essential components is the electrolyte, which in the case of thin, flexible batteries, is often in the form of a hydrogel. These flexible, water-containing materials absorb dissolved sodium salts and can conduct ions.
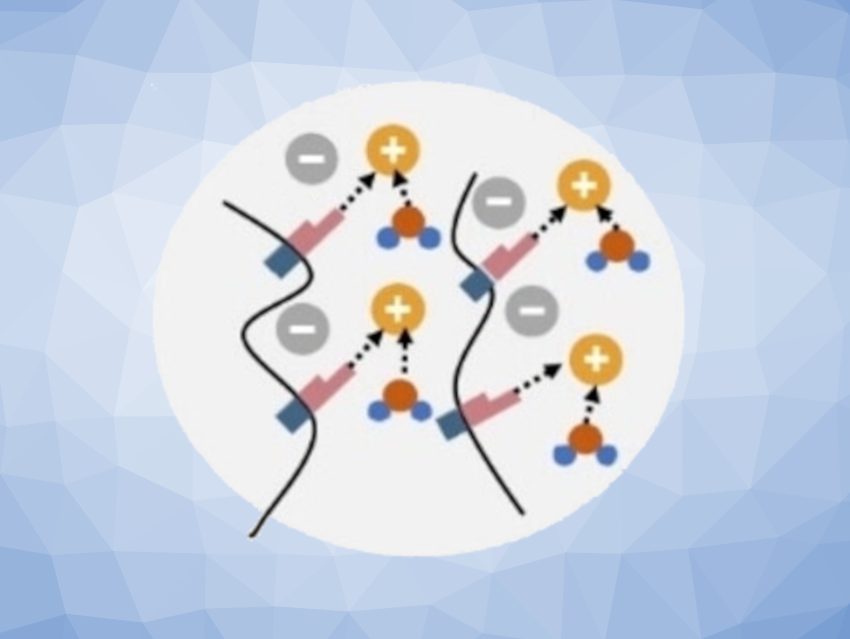
Despite the suitability of hydrogels, an as-yet unsolved problem is phase separation and salting out at the high salt concentrations needed for a broad electrochemical stability window. The team succeeded in modifying a hydrogel for a sodium-ion battery to make it absorb considerably more salt in a stable and secure manner.
Polymer Methylation
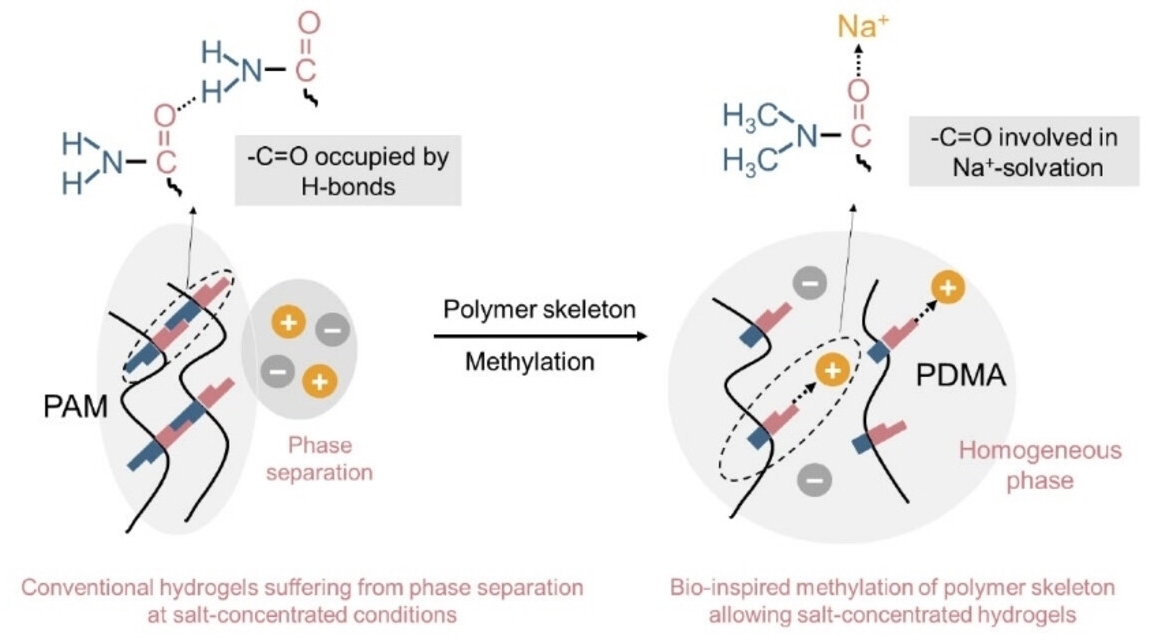
To achieve this, the researchers turned to a technique also employed in nature for the regulation of water- and salt-binding in large biomolecules: methylation. In proteins, methylation causes the “capping” of amine and amide groups, which become less accessible for water molecules that play a role in cross-linking within the protein structure and the dissolution of salt ions.
As the polyamide polymers used for hydrogels also contain amide groups, their extensive cross-linking through water molecules can cause salting out, which leads to the breakdown of the electrolyte (pictured below on the left). With this in mind, the team compared a hydrogel made of a common polyamide to a hydrogel made of a polyamide with methylated amide groups (pictured below on the right). The latter was able to absorb significantly more salt than the original variant. Even at record-high salt concentrations, the hydrogel electrolyte remained transparent and stable.
Stable, Concentrated Electrolytes Improve Performance
An unconventionally salt-concentrated hydrogel electrolyte reaching a salt fraction up to 44 mol%. The higher salt content means that the electrochemically usable voltage range of the cell can be expanded. In addition, the team did not observe any signs of disintegration at the electrodes and better cycling stability, and the assembled battery cell achieved a greater capacity than the non-methylated variant. It was even possible to use inexpensive aluminum foil as a current collector in this system.
The researchers suggest that simple polyamide methylation could also be suitable for other technologies, for example, in drug development, to make hydrogels more resistant to salts and, therefore, more stable.
- A Bio‐Inspired Methylation Approach to Salt‐Concentrated Hydrogel Electrolytes for Long‐Life Rechargeable Batteries,
Tingting Liu, Xiaofan Du, Han Wu, Yongwen Ren, Jinzhi Wang, Hao Wang, Zheng Chen, Jingwen Zhao, Guanglei Cui,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202311589