Multidrug-resistant bacterial infections that cannot be treated by known antibiotics pose a serious health threat. Yiyun Cheng, East China Normal University, Shanghai, China, and colleagues have developed a method for the development of new antimicrobials to fight resistant pathogens. The candidate compounds are based on protein building blocks with fluorous lipid chains.
Finding a Way Around Drug Resistances
Antibiotics are often prescribed far too readily, sometimes distributed without prescriptions, and used prophylactically in factory farming to prevent infections. As a result, resistances are on the rise—increasingly against reserve antibiotics, as well. The development of innovative alternatives to traditional antibiotics is, thus, essential.
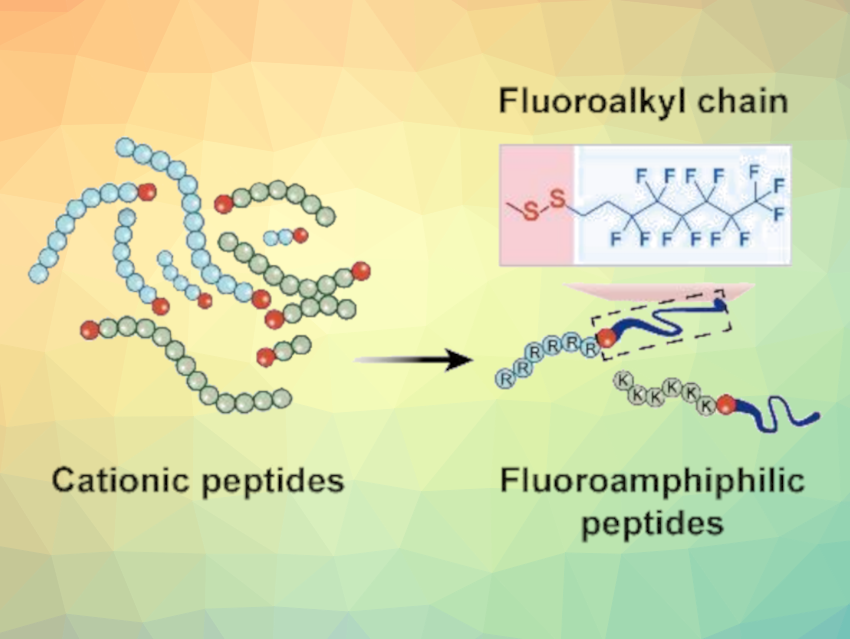
It is possible to learn some lessons from the microbes themselves. Lipoproteins, small protein molecules with fatty acid chains, are widely used by bacteria in their battles against microbial competitors. A number of lipoproteins have already been approved for use as drugs. The common factors among the active lipoproteins include a positive charge and an amphiphilic structure, meaning they have both lipophilic and hydrophilic parts. This allows them to bind to bacterial membranes and “pierce” through them to the interior.
Fluorine Can Improve the Activity of Lipopeptides
The team aimed to amplify this effect by replacing hydrogen atoms in the lipid chain with fluorine atoms. These make the lipid chain simultaneously hydrophobic and lipophobic. Their particularly low surface energy strengthens their binding to cell membranes, while their lipophobicity disrupts the cohesion of the membrane.
The researchers synthesized a library of fluorous lipopeptides from fluorinated hydrocarbons and peptide chains. To link the two pieces, they used the amino acid cysteine, which binds them together via a disulfide bridge (see picture).
The team screened the candidates by testing their activity against methicillin-resistant Staphylococcus aureus (MRSA), a widespread, dangerous strain of bacteria that is resistant to nearly all antibiotics. The most effective compound they found was “R6F”, a fluorous lipopeptide made of six arginine units bound to a C8F13 lipid chain. To increase biocompatibility, R6F was enclosed within phospholipid nanoparticles.
High Therapeutic Efficacy and Minimal Adverse Effects
In mouse models, the resulting R6F nanoparticles were shown to be very effective against sepsis and chronic wound infections by MRSA. No toxic side effects were observed.
The nanoparticles seem to attack the bacteria in several ways: They inhibit the synthesis of important cell-wall components, promoting collapse of the walls. They also pierce the cell membrane and destabilize it, disrupt the respiratory chain and metabolism, and increase oxidative stress, while simultaneously disrupting the antioxidant defense system of the bacteria. In combination, these effects kill the bacteria and no resistance appeared to develop.
Overall, these insights could provide starting points for the development of highly efficient fluorous peptide drugs to treat multi-drug resistant bacteria.
- A Fluorous Peptide Amphiphile with Potent Antimicrobial Activity for the Treatment of MRSA‐induced Sepsis and Chronic Wound Infection,
Jingjing Hu, Nan Liu, Qianqian Fan, Yunqing Gu, Sijia Chen, Fang Zhu, Yiyun Cheng,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202403140