Piperidines are cyclic amines with a six-membered ring. These heterocycles are useful, e.g., in pharmaceutical chemistry. However, their targeted functionalization can be somewhat challenging, which is an issue, for example, for structure–activity relationship studies. For the aromatic counterparts of piperidines, i.e., pyridines, there is a larger number of predictable transformations.
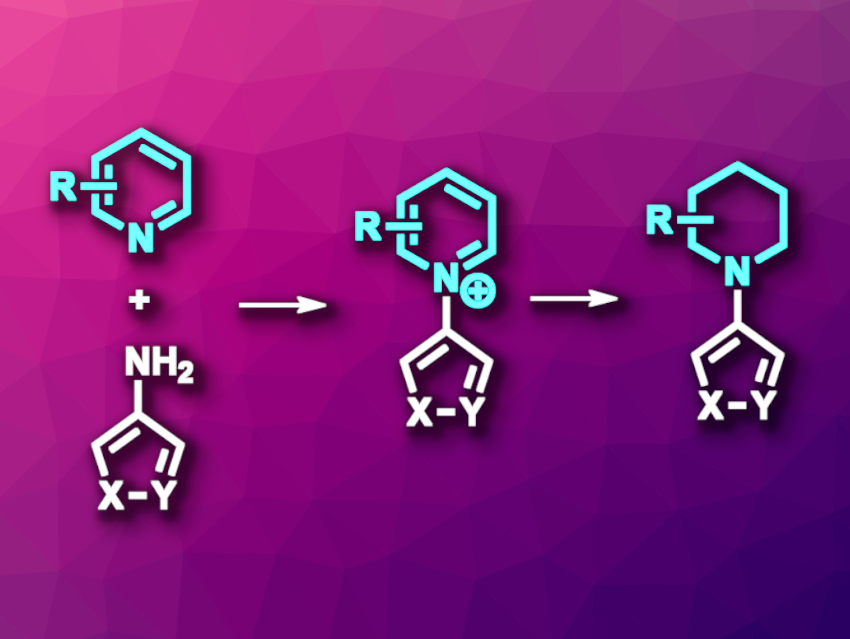
Eric M. Phillips, Merck & Co., Inc., Rahway, NJ, USA, Andrew McNally, Colorado State University, Fort Collins, USA, and colleagues have developed a general approach to synthesizing N-(hetero)arylpiperidines, which uses a ring-opening and ring-closing approach to first give N-(hetero)arylpyridinium salts as intermediates that can then be used to synthesize a wide variety of piperidine derivatives. The team synthesized ring-opened imines from pyridine derivatives using Tf2O and collidine or 2,6-di-tert-butyl-4-methylpyridine (DTBMP), together with (heteroaryl)anilines as nucleophiles.
The ring-opened imine intermediates were then directly transformed into pyridinium salts via ring-closing in the presence of AcOH at 70 °C. This one-pot approach gave the desired pyridinium salts in moderate to good yields. The team then synthesized a variety of piperidine derivatives from the pyridinium salts via reduction reactions (including asymmetric variants) and nucleophilic addition reactions. According to the researchers, the developed approach could be useful for generating compound libraries for structure–activity relationship studies.
- A General Strategy for N-(Hetero)arylpiperidine Synthesis Using Zincke Imine Intermediates,
Jake D. Selingo, Jacob W. Greenwood, Mary Katherine Andrews, Chirag Patel, Andrew J. Neel, Barbara Pio, Michael Shevlin, Eric M. Phillips, Matthew L. Maddess, Andrew McNally,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c11504