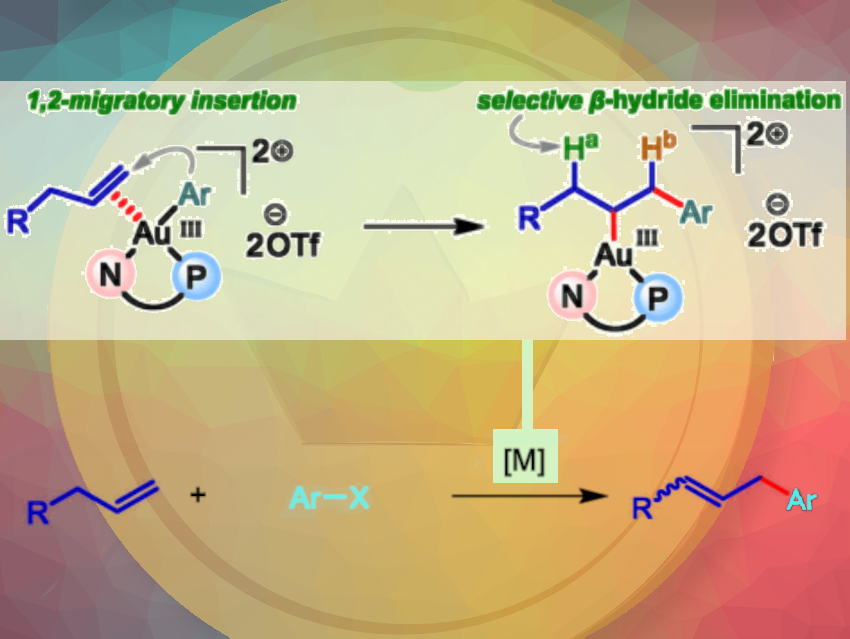
The transition metal-catalyzed Heck reaction has become a crucial tool in modern organic synthesis since its discovery. However, the reaction has certain limitations such as requiring specialized substrates and resulting in a mixture of regioisomeric products due to the undesirable chain-walking process.
Vincent Gandon, Paris-Saclay University, Orsay, France, Nitin T. Patil, Indian Institute of Science Education and Research Bhopal, India, and colleagues have described a gold-catalyzed Heck reaction that uses the ligand-enabled Au(I)/Au(III) redox catalysis (pictured). This is the first time that gold chemistry enables the elementary organometallic steps such as migratory insertion and β-hydride elimination. This methodology overcomes the limitations of previously known transition metal-catalyzed Heck reactions. Long-chain aliphatic alkenes can be used without the need for a preinstalled directing/chelating group.
The team discovered that the reaction offers complementary regioselectivity compared to other transition metal catalysis. For instance, the Au(III)–alkyl intermediate generated in situ after migratory insertion could selectively undergo β-hydride elimination with Ha over Hb, favoring the formation of allylic products over styrenyl products. This reversal of regioselectivity for electronically unbiased alkenes has not been reported before. To understand the origin of the regioselectivity, the team conducted DFT studies and such with deuterated alkenes. They revealed that strong κ1-C coordination between gold and the ipso aryl carbon in the transiiton state is crucial for obtaining the allylic product.
The researchers belive that their findings should open up several avenues for accessing novel reactivities and selectivities in organometallic chemistry.
- Gold-Catalyzed Heck Reaction,
Vivek W. Bhoyare, E. Daiann Sosa Carrizo, Chetan C. Chintawar, Vincent Gandon, Nitin T. Patil,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c02544