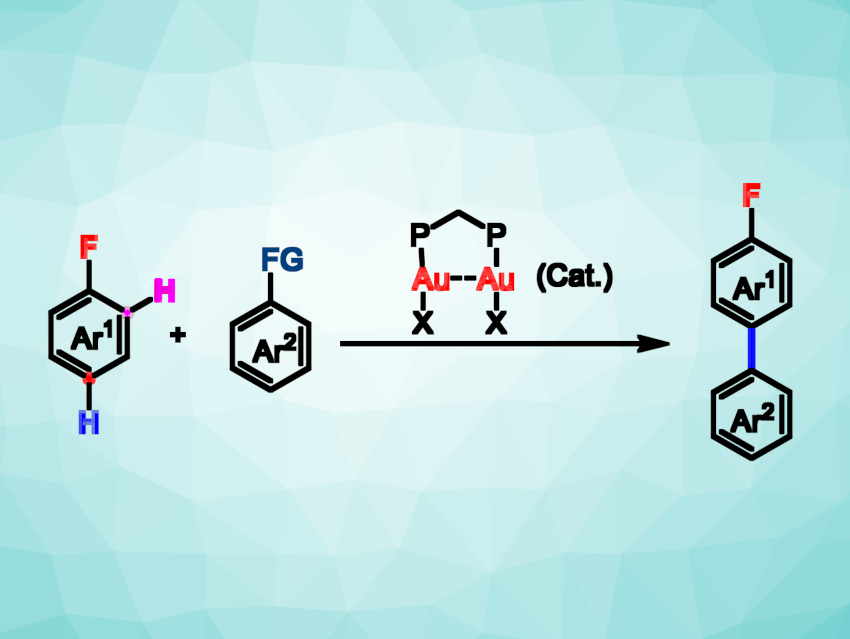
The direct activation of C–H bonds in aromatic compounds can be a useful strategy for forming C–C bonds and constructing organic compounds with uses, e.g., in pharmaceutical chemistry, agrochemistry, or polymer chemistry. However, controlling the regioselectivity of this type of reaction can be challenging. Directing groups or sterically bulky groups can be used to control the reaction, but this requires extra steps. Alternatively, electron-withdrawing groups such as NO2 can steer the reaction, while the weakly deactivating fluorine substituent, for example, generally result in lower selectivity. The para-selective C–H arylation of monofluoroarenes, in particular, can be difficult to achieve.
Jin Xie, Nanjing University, China, and colleagues have developed a method for the para-selective C–H arylation of a wide array of monofluoroarenes. They use a dinuclear gold catalyst and readily available aromatic silanes and germanes as coupling partners (reaction pictured, FG = R3Si, R3Ge). The team used dppm(AuOTs)2 (dppm = bis(diphenylphosphino)methane) as the catalyst and hexafluorobenzene as the solvent, conducting the reactions at room temperature.
The desired products were obtained with high para-selectivity (up to 98:2). The reaction has a good functional group tolerance and a broad substrate scope, with over 80 different products synthesized. The team performed mechanistic experiments and quantum-chemical calculations and found that the para-selectivity is based on the electronic effects and ligand flexibility of the dinuclear gold catalyst, along with a noncovalent interaction with the solvent.
- Dinuclear Gold‐Catalyzed para‐Selective C‐H Arylation of Undirected Arenes by Noncovalent Interactions,
Duan-Yang Liu, Jie Han, Kai Liu, Yaohang Cheng, Hairen Tan, Xiaoliang Yang, Weipeng Li, Jin Xie,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202313122