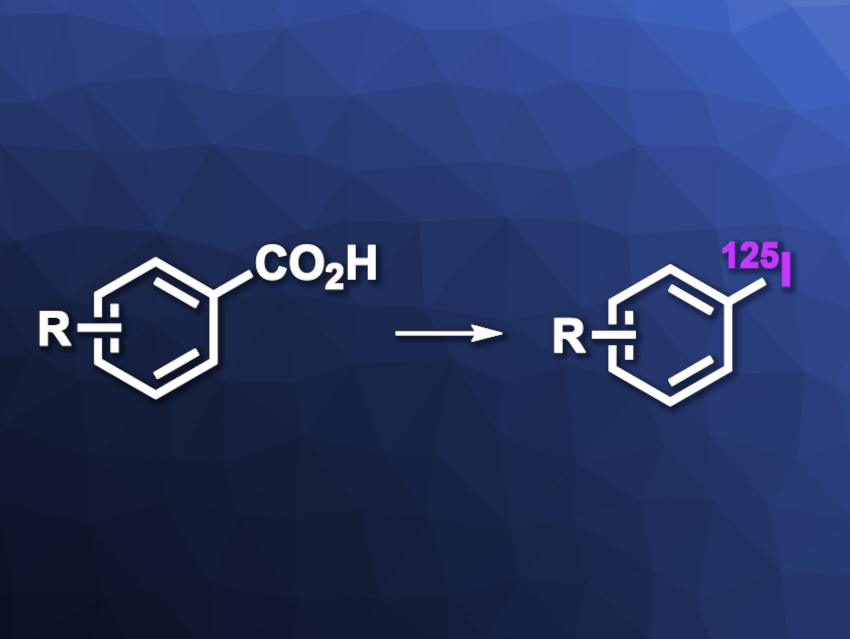
Labeling molecules with radioactive isotopes is an important tool in the life sciences and nuclear medicine. Radiolabeling enables, e.g., the tracking of chemical species, radiotherapy, or medical imaging. The radioiodination of arenes, for example, gives useful tracers for different applications. Existing radioiodination approaches, however, have some drawbacks such as the use of fragile precursors.
Thomas Cailly, Normandie Université, Caen, France, and colleagues have developed a radioiodination approach that uses stable carboxylic acids as precursors. The team first reacted a variety of benzoic acid derivatives (as well as carboxylic acid-functionalized heteroarenes, cinnamic acid, and phenylpropiolic acid) with PPh3AuCl and Ag2O in dimethylformamide (DMF) at 100 °C to give organogold(I) intermediates of the type Ar–AuPPh3. This was followed by the addition of [125I]NIS (NIS = N-Iodosuccinimide) at room temperature to give the desired radioiodinated arenes.
The radiolabeled products were obtained in moderate to high yields. Depending on the substrates, the decarboxylation conditions were varied to optimize yields: For reactants such as 4-nitro-, 4-cyano-, 4-trifluoromethyl-, or 4-acetylbenzoic acid, the team used PPh3AuCl, Cu2O, and 1,10-phenanthroline in a solvent mixture of N-methyl-2-pyrrolidone (NMP) and quinoline at 150 °C under microwave irradiation. The crude gold(I) organometallic solution was shelf stable for at least 33 days, which makes the approach convenient for the radioiodination of a broad spectrum of (hetero)arenes.
- Gold(I)-Mediated Radioiododecarboxylation of Arenes,
Hugo Bloux, Ahmed Ait Khouya, Jana Sopkova-de Oliveira Santos, Frédéric Fabis, Emmanuelle Dubost, Thomas Cailly,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c03191