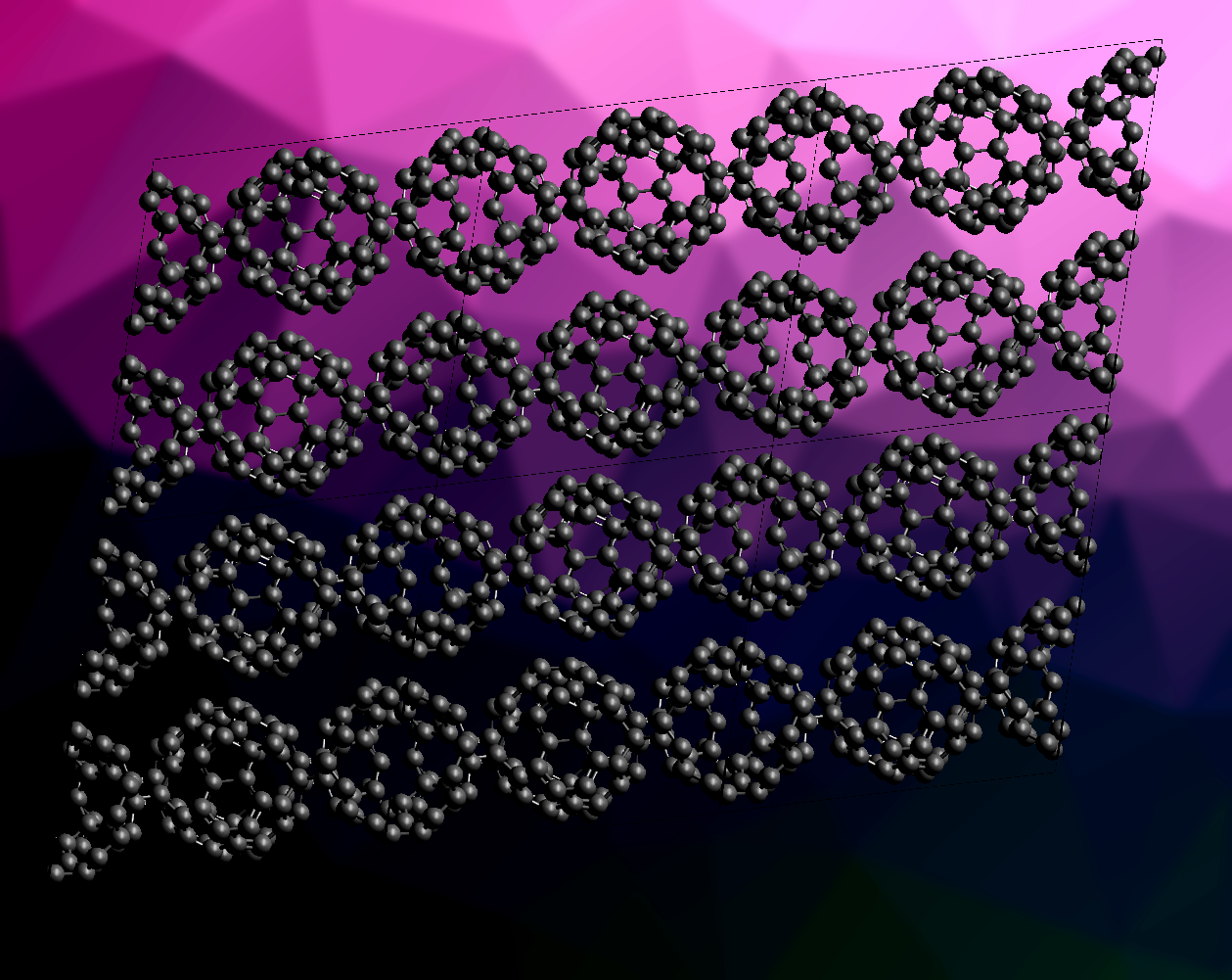
Carbon has a variety of allotropes, such as diamond, graphite, graphene, and different fullerenes. Graphene features two-dimensional sheets of carbon atoms, while the fullerene C60 has a ball-like shape composed of fused hexagons and pentagons.
Elena Meirzadeh, Michael L. Steigerwald, Jingjing Yang, Colin Nuckolls, Xavier Roy, Columbia University, New York, NY, USA, and colleagues have synthesized a new carbon allotrope that combines structural elements of graphene and C60, which the team has called “graphullerene” (pictured). This allotrope is a two-dimensional sheet of fullerene units, which are arranged hexagonally. The team first grew single crystals of the magnesium-doped polyfulleride (Mg4C60)∞. For this, they mixed Mg powder and C60 and pressed the mixture into a pellet, which was sealed in a silica tube and subjected to a temperature gradient in a furnace. The desired product was obtained in the form of black crystals at the colder end of the tube.
The resulting (Mg4C60)∞ was characterized using single-crystal X-ray diffraction. The team found a layered structure with a quasi-hexagonal lattice. Each C60 unit forms eight covalent σ bonds to six neighbors: four single connections to another C60 and two pairs of bonds to the other two neighboring units. The layers are only weakly bonded.
The researchers then suspended (Mg4C60)∞ in dilute aqueous solutions of acetic acid or nitric acid to remove most of the magnesium, followed by suspending the crystals in N-methylpyrrolidone at 180 °C to completely remove the remaining Mg. The resulting graphullerite crystals (named analogous to graphite) were mechanically exfoliated to obtain few-layer samples of graphullerene. The team obtained flakes as thin as bilayers. They found that the thermal conductivity of graphullerene is much higher than that of C60, probably due to the in-plane covalent bonding. According to the researchers, the work indicates that there could be an entire family of “superatomic” allotropes of carbon.
- A few-layer covalent network of fullerenes,
Elena Meirzadeh, Austin M. Evans, Mehdi Rezaee, Milena Milich, Connor J. Dionne, Thomas P. Darlington, Si Tong Bao, Amymarie K. Bartholomew, Taketo Handa, Daniel J. Rizzo, Ren A. Wiscons, Mahniz Reza, Amirali Zangiabadi, Natalie Fardian-Melamed, Andrew C. Crowther, P. James Schuck, D. N. Basov, Xiaoyang Zhu, Ashutosh Giri, Patrick E. Hopkins, Philip Kim, Michael L. Steigerwald, Jingjing Yang, Colin Nuckolls, Xavier Roy,
Nature 2023, 613, 71–76.
https://doi.org/10.1038/s41586-022-05401-w