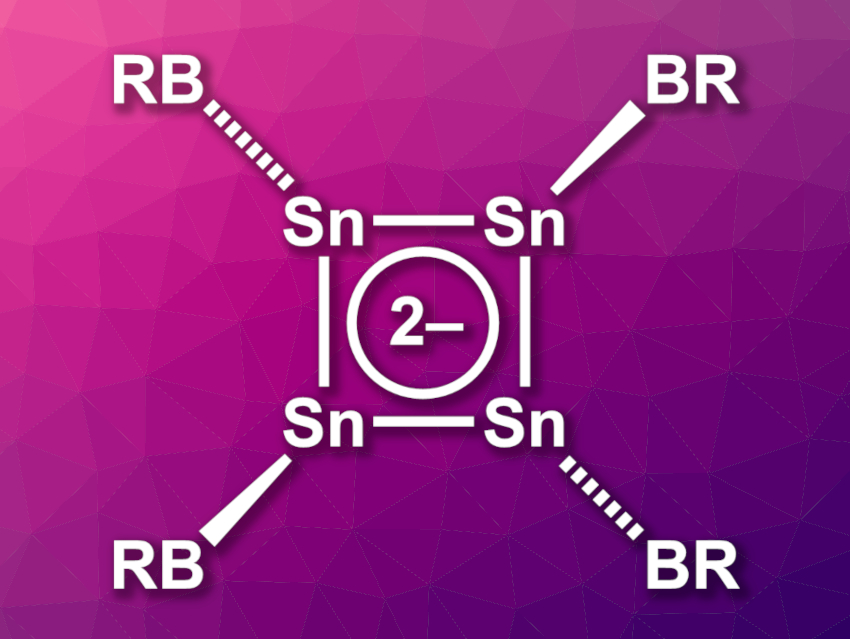
Aromatic compounds are widely used in organic chemistry. Cylobutadiene has 4 π electrons, so according to Hückel’s rule, its dianion with 6π electrons should be aromatic. Analogues of such compounds with heavier group 14 elements in place of carbon can be challenging to synthesize. Some cyclobutadiene dianion analogues with silicon and/or germanium atoms are known, unlike a tin-containing analogue.
Simon Aldridge, University of Oxford, UK, and colleagues have synthesized the first tin-containing analogue of the cyclobutadiene dianion, [Sn4{B(NDippCH)2}4]2– (pictured, R = (NDippCH)2, Dipp = 2,6-iPr2C6H3). The team started from the boryltin(II) precursor {(HCDippN)2B}Sn(IPrMe)Br (IPrMe = C{(NiPrCMe)2}), which was reduced using potassium graphite to give the cyclic tetratin tetraboryl system K2[Sn4{B(NDippCH)2}4].
The compound was characterized using X-ray crystallography and features a nearly square arrangement of the tin atoms in a puckered ring. Quantum chemical calculations indicate that the system is non-aromatic. The dianion can be protonated using benzoic acid to obtain the neutral dihydride Sn4{B(NDippCH)2}4H2, which can be considered a heavier homologue of cyclobutene.
- Synthesis of homo-metallic heavier analogues of cyclobutene and the cyclobutadiene dianion,
Xiongfei Zheng, Agamemnon Crumpton, Andrey Protchenko, Mathias Ellwanger, Andreas Heilmann, Simon Aldridge,
ChemRxiv 2022.
https://doi.org/10.26434/chemrxiv-2022-4nq1v
The research has been published as a preprint and has not yet been peer-reviewed.