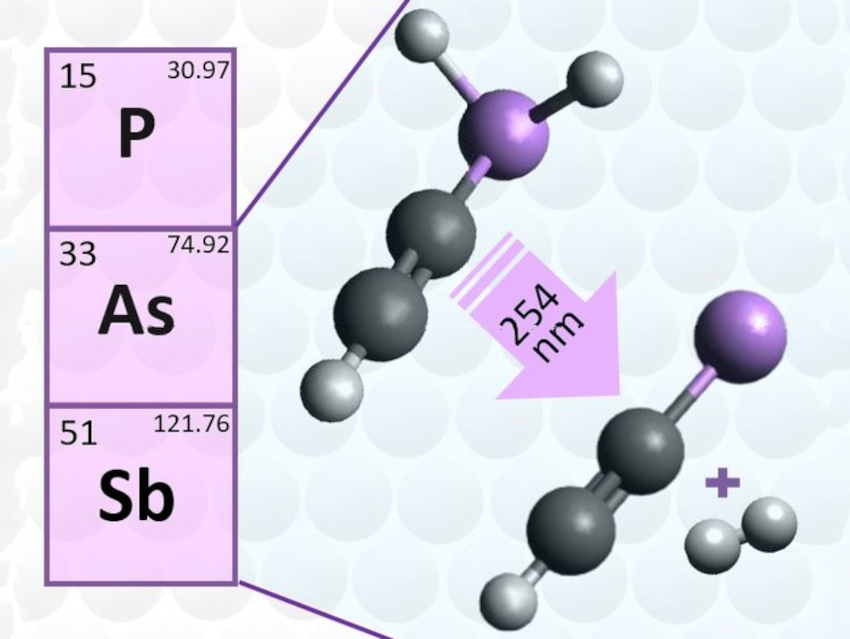
There have been several attempts to synthesize free arsinidines and stibinidenes, i.e., molecules with a univalent arsenic or antimony atom. The only such species reported so far were simple by-products of gas-phase decompositions: AsH, SbH, and CH3As. Such molecules are heavier pnictogen analogues of nitrenes, generally with a triplet ground state.
Arun-Libertsen Lawzer, Institute of Physical Chemistry, Polish Academy of Sciences, Warsaw, and colleagues have photochemically generated ethynylarsinidene (HCCAs) and ethynylstibinidene (HCCSb) in an argon matrix through a photodehydrogenation process (pictured). The team used HCCAsH2 and HCCSbH2 as precursors. They were obtained from the respective ethynyldichloro derivatives using tributyltin as a reducing agent and then mixed with argon, trapped at 6 K, and subjected to UV photolysis.
Triplet HCCAs and HCCSb were the main products identified, using a combination of IR spectroscopy and quantum chemical calculations. A comparison of the electronic structures of HCCX molecules (X = N, P, As, or Sb) indicates that the univalent character of the pnictogen atom is more pronounced in HCCAs and HCCSb than in HCCP, while HCCN has a predominantly cyanocarbene structure instead of a nitrene structure.
The researchers propose that the fact that they did not observe HCCAsH and HCCSbH radicals suggests that HCCAs and HCCSb are formed through a concerted loss of H2 and that the photodehydrogenation reaction might be a viable method for generating these species in solution and stabilizing them with a suitable ligand.
- Free ethynylarsinidene and ethynylstibinidene: Heavier analogues of nitrenes and phosphinidenes,
Arun-Libertsen Lawzer, Elavenil Ganesan, Marcin Gronowski, Thomas Custer, Jean-Claude Guillemin, Robert Kołos,
Chem. Eur. J. 2023.
https://doi.org/10.1002/chem.202300887