Azobenzene derivatives (A–N=N–Ar) are widely used, e.g., as photoswitches, and their chemistry is well understood. Heavier dipnictenes such as diphosphenes are generally more difficult to synthesize and require sterically demanding substituents for stabilization. Distibenes and dibismuthenes are less common as they require even more complex substituents. Compared to azobenzenes and diphosphenes, the activation of the double bond in the resulting distibenes and dibismuthenes is very challenging, resulting in poor reactivity towards both organic compounds and transition metals.
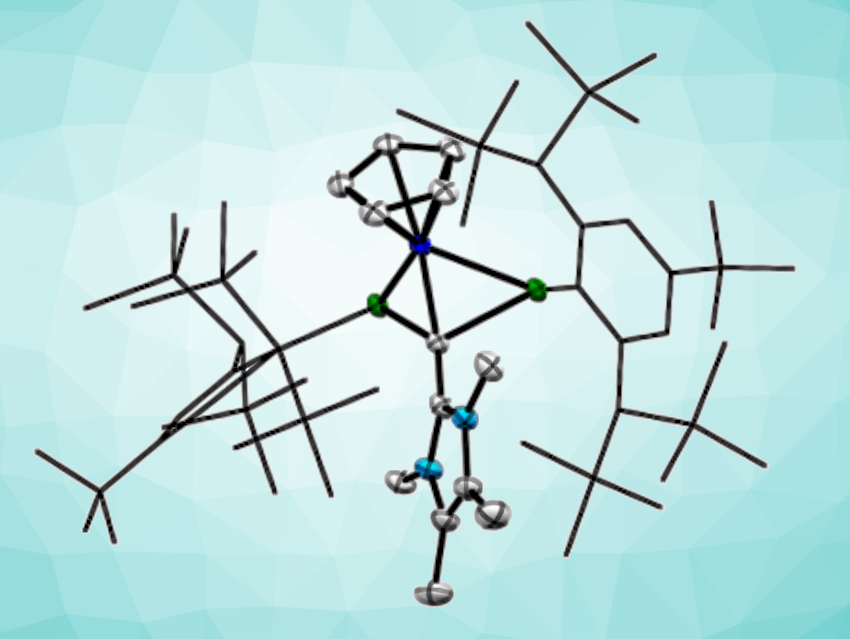
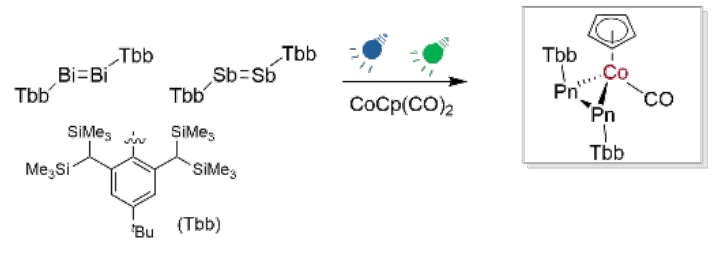
Alessandro Bismuto, University of Bonn, Germany, Antonio Frontera, University of the Balearic Islands, Palma, Spain, and colleagues have developed a protocol that uses visible light to “unlock” the reactivity of heavy dipnictenes. The team first prepared Tbb–Sb=Sb–Tbb and Tbb–Bi=Bi–Tbb (Tbb = 2,6-[CH(SiMe3)2]2-4-(tBu)C6H2). These dipnictenes were reacted with CpCo(CO)2 (Cp cyclopentadienyl), under blue LED light (451 nm) for the distibene and under green LED light (521 nm) for the dibismuthene. The team isolated unique dipnictene-cobalt complexes featuring a CoPn2 metallacyclic unit (pictured below, Pn = Bi, Sb).
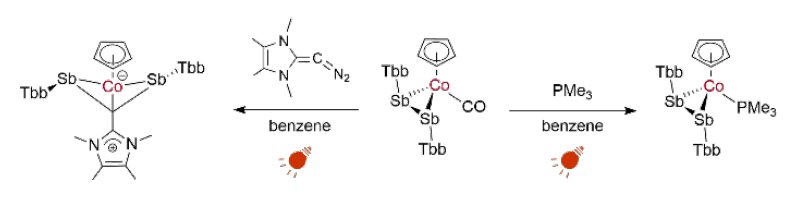
Using another light activation with red light (625 nm) to enhance its reactivity, the antimony complex can undergo a ligand exchange (pictured below on the right). In addition, the same wavelength of light can also be used to promote the insertion of an N-heterocyclic vinylidene unit into the Sb–Sb bond, accessing an unusual four-membered bicyclo[1.1.0]butane analog containing only a single carbon atom (pictured below on the left).
- Light‐dependent Reactivity of Heavy Pnictogen Double Bonds,
Daniel Meleschko, Prasenjit Palui, Rosa M. Gomila, Gregor Schnakenburg, Alexander C. Filippou, Antonio Frontera, Alessandro Bismuto,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202405400