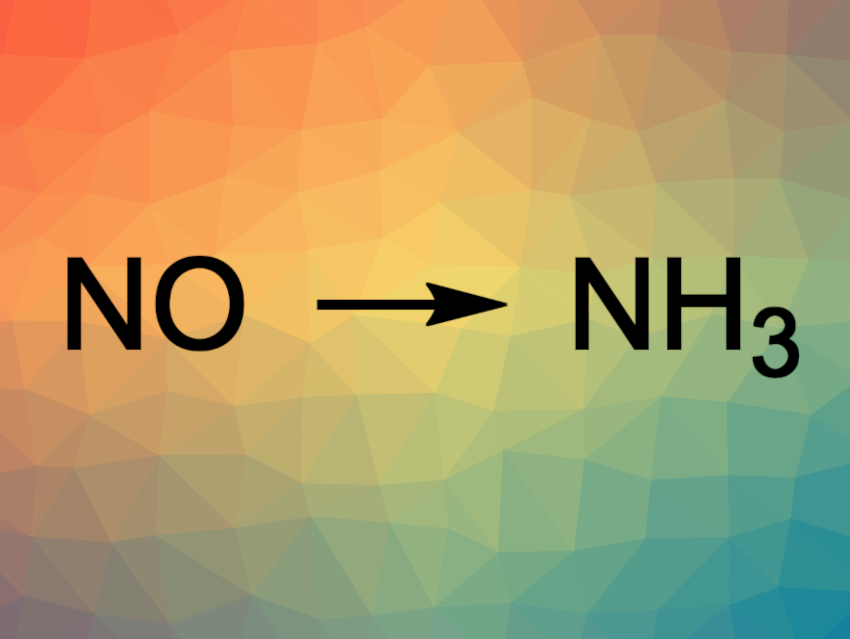
Nitric oxide (NO) is an air pollutant, e.g., in car exhaust gas. Converting it into the relatively inert N2 or even valuable products such as ammonia is, thus, useful. Green and sustainable electrochemical alternatives to the energy-intensive process for industrial ammonia production are attractive research targets. The electrochemical reduction of N2 is one approach, but the chemical inertness of N2 makes this challenging. The electrocatalytic reduction of NO could be promising for the synthesis of NH3 due to the easier activation of NO.
Chen Chen, Shuangyin Wang, Hunan University, Changsha, China, Chandra Veer Singh, University of Toronto, Canada, and colleagues have prepared hexagonal-close-packed cobalt (hcp-Co) nanosheets to be used as catalysts for NO electroreduction. The team synthesized hcp-Co sheets from cobalt(II)chloride via a hydrothermal method. Scanning electron microscopy (SEM) showed that the hcp-Co catalyst has a nanoflower morphology composed of many small nanosheets.
The researchers evaluated the electrochemical NO reduction performance of hcp-Co in an aqueous solution of Na2SO4, with the catalyst powder loaded onto carbon paper and used as the working electrode. The catalyst showed a high ammonia yield of 439.50 μmol cm–2 h–1 and a Faraday efficiency of 72.58 %. Co nanosheets with the face-centered cubic phase (fcc-Co) showed significantly lower performance than hcp-Co. The team performed density functional theory (DFT) calculations and desorption experiments to gain further insights. Based on the results, they propose that the higher activity of hcp-Co compared with fcc-Co can be attributed to enhanced NO adsorption and improved proton diffusion.
- Hexagonal Cobalt Nanosheets for High-Performance Electrocatalytic NO Reduction to NH3,
Dongdong Wang, Zhi-Wen Chen, Kaizhi Gu, Chen Chen, Yingying Liu, Xiaoxiao Wei, Chandra Veer Singh, Shuangyin Wang,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c00276