Solid superbases can act as heterogeneous catalysts for a variety of reactions, e.g., transesterifications, alkylations, or isomerizations, under mild conditions. However, available solid superbases suffer from limitations such as aggregation and the leaching of active sites. This results in a low utilization of active sites and poor stability during recycling.
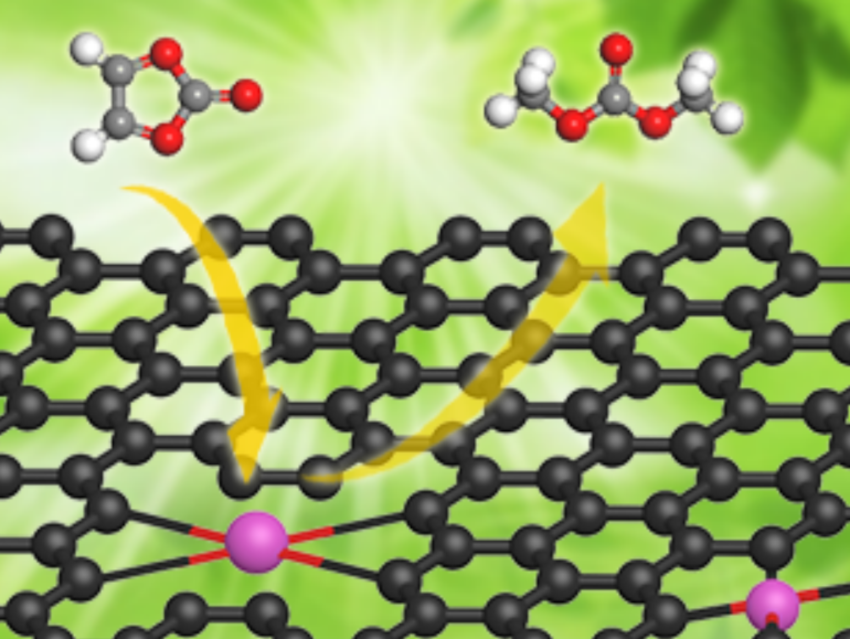
Lin-Bing Sun, Nanjing Tech University, China, You Han, Tianjin University and Haihe Laboratory of Sustainable Chemical Transformations, Tianjin, China, and colleagues have developed new solid superbases that feature potassium single atoms on graphene. These superbases were prepared via a tandem redox strategy. An initial redox reaction between KNO3 as a potassium source and the graphene support at around 200–400 °C gives K2O, as well as NO and CO2 as gaseous products. In a second redox reaction at about 800 °C, the graphene further reduces K2O to form potassium single atoms on graphene (K1/G).
In the K1/G system, some K atoms are well separated from each other, while others are close to each other (neighboring single atoms or NSAs). The potassium NSAs exhibit superbasicity. According to the researchers, K1/G is highly active in the synthesis of dimethyl carbonate via transesterification (pictured) because of the high dispersion of such superbasic sites. The turnover frequency of the catalyst is much higher than that for other reported catalysts, and it is stable when recycled.
- Catalytically stable potassium single‐atom solid superbases,
Song-Song Peng, Xiang-Bin Shao, Meng-Xuan Gu, Guo-Song Zhang, Chen Gu, Yao Nian, Yiming Jia, You Han, Xiao-Qin Liu, Lin-Bing Sun,
Angew. Chem. Int. Ed. 2022.
https://doi.org/10.1002/anie.202215157