Borrowing hydrogen (BH) reactions have garnered research attention over the past decade as an environmentally friendly option for the synthesis of C–C and C–N bonds. The strategy typically relies on three steps: a dehydrogenation, an intermediate reaction, and a hydrogenation. These reactions commonly employ catalysts based on transition metals, in conjunction with either stoichiometric or catalytic amounts of added base, and generate only water as byproduct. The method can, for example, be used for the β-alkylation of secondary alcohols with primary alcohols or the N-alkylation of amines with alcohols.
Ola F. Wendt, Lund University, Sweden, Magnus T. Johnson, Lund University and Perstorp AB, Perstorp, Sweden, and colleagues have found that these two types of catalytic transformations often conducted via a borrowing-hydrogen approach—the N-alkylation of amines with alcohols and the β-alkylation of secondary alcohols with primary alcohols—can be performed efficiently using only a catalytic amount of base under air without the need for transition metal-based catalysts.
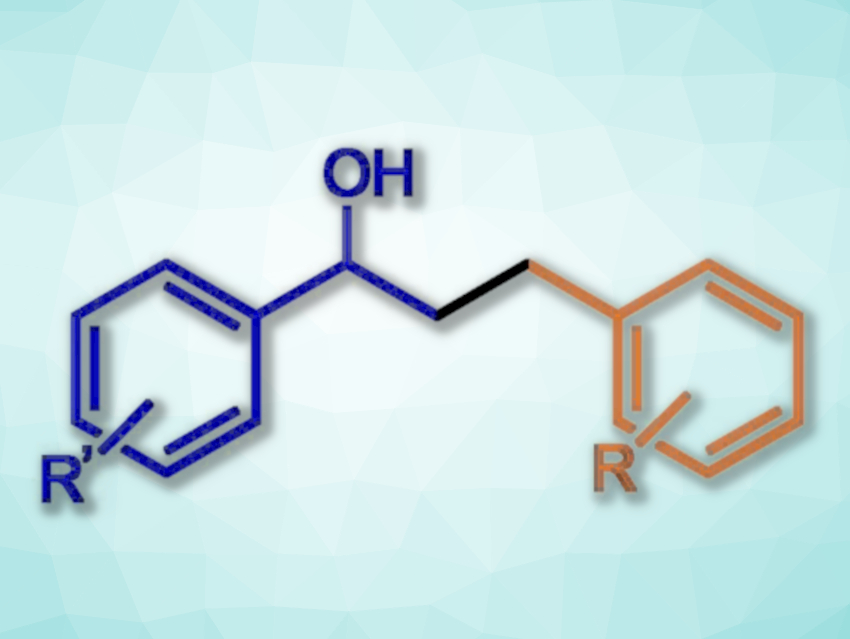
For the N-alkylation of amines with alcohols, the team reacted aniline derivatives with, e.g., benzyl alcohols in the presence of KOtBu as the base, using toluene as the solvent. The reactions were performed at 140 °C. For the β-alkylation of secondary alcohols with primary alcohols (example product structure pictured), KOH was used as the base, and the reactions were performed at 120 °C.
The method showed a broad substrate scope for both types of reactions, with yields up to 98 %. The team proposes a reaction mechanism that is based on air oxidation of the alcohol to an aldehyde, followed by condensation to an unsaturated intermediate that undergoes transfer hydrogenation with alcohol to give the desired product.
- Highly Efficient Base Catalyzed N‐Alkylation of Amines with Alcohols and β‐Alkylation of Secondary Alcohols with Primary Alcohols,
Nitish K. Garg, Mattias Tan, Magnus T. Johnson, Ola F. Wendt,
ChemCatChem 2023.
https://doi.org/10.1002/cctc.202300741