Ligands with high steric bulk can be useful, e.g., in transition-metal catalysis or in stabilizing unusual main-group species. IPr* (1,3-bis(2,6-bis(diphenylmethyl)-4-methylphenyl)imidazol-2-ylidene), for example, is a highly sterically hindered and flexible N-heterocyclic carbene (NHC) ligand. Cyclic (alkyl)(amino)carbenes (CAACs) are electron-rich carbon analogues of NHCs.
Michal Szostak, Rutgers University, Newark, NJ, USA, and colleagues have combined the properties of bulky IPr* with those of CAAC ligands and synthesized IPr*–CAAC ligands and complexes (example pictured). The team started from anilines, which were functionalized with diphenylmethyl groups and reacted with propanal derivatives to obtain imines. The imines underwent an α-alkylation, followed by an intramolecular cyclization to give the HBPh4 salts of the desired IPr*–CAAC ligands. The team used a methyl substituent or a more electron-rich methoxy group at the aniline and either methyl groups or more bulky cyclohexyl units at the five-membered ring of the ligands.
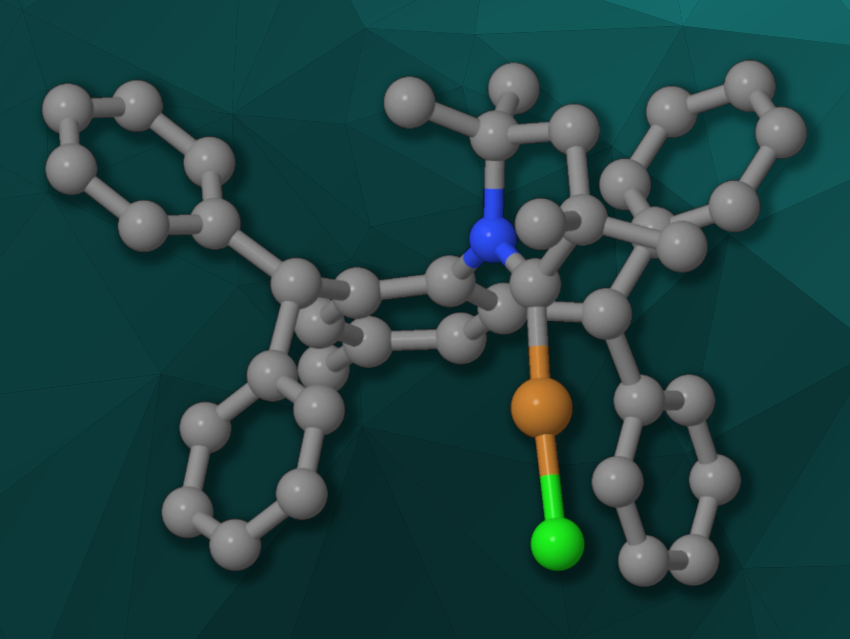
When the precursor salts of the ligands were reacted with CuCl, the team observed the formation of [Cu(IPr*–CAAC)Cl] complexes (example pictured). The researchers investigated the activity of the [Cu(IPr*–CAAC)Cl] complexes in the copper-catalyzed hydroboration of alkynes. They found that the efficiency and regioselectivity were significantly improved compared with the parent CAACs. The team’s approach could provide a useful strategy for the development of other electron-rich, flexible, and bulky ligands with uses in catalysis.
- CAAC–IPr*: Easily Accessible, Highly Sterically-Hindered Cyclic (Alkyl)(Amino)Carbenes,
Wenchao Chu, Tongliang Zhou, Elwira Bisz, Blazej Dziuk, Roger A Lalancette, Roman Szostak, Michal Szostak,
Chem. Commun. 2022.
https://doi.org/10.1039/D2CC05668B