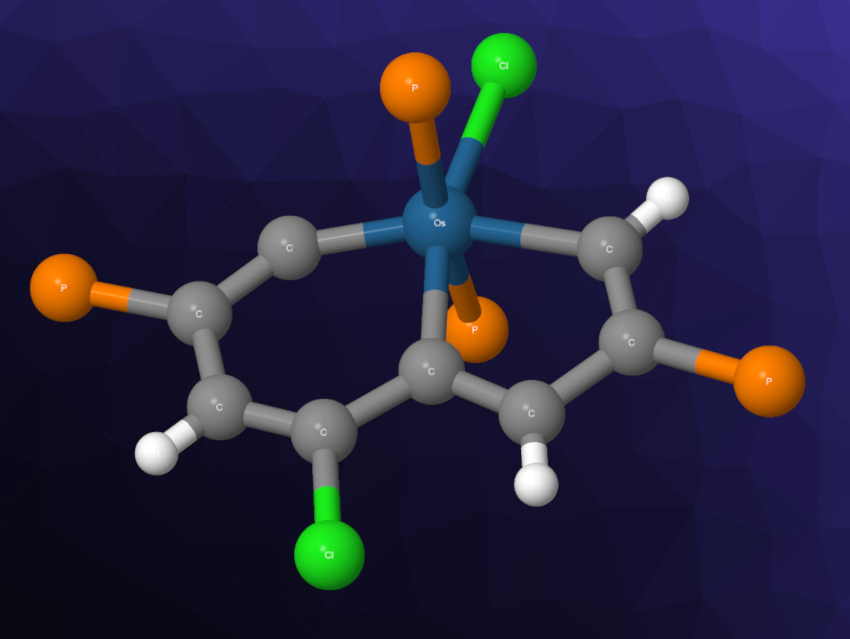
The realization of highly strained rings, such as those containing cyclic allenes or metal vinylidenes, is an interesting research target due to the destabilizing effect of the ring strain. Antiaromatic compounds are also destabilized. Thus, synthesizing strained, antiaromatic systems with both of these destabilizing features is a considerable challenge.
Haiping Xia, Xiamen University, China, and Southern University of Science and Technology, Shenzhen, China, and colleagues have isolated a highly strained antiaromatic metallacycle (I, simplified structure pictured above), which features the smallest angle of a metal vinylidene within a six-membered ring (147.4°) and which combines antiaromaticity with a highly strained metallacycle.
The team prepared the metallacycle from a precursor with multiple alkyne groups, which was reacted with OsCl2(PPh3)3 in the presence of triphenylphosphine (PPh3) in dichloromethane (DCM), followed by the addition of HCl·Et2O. The structure of the product was confirmed by single-crystal X-ray diffraction. According to the researchers, the metal incorporation and the phosphonium substituents play a crucial role in the stabilization of the compound.
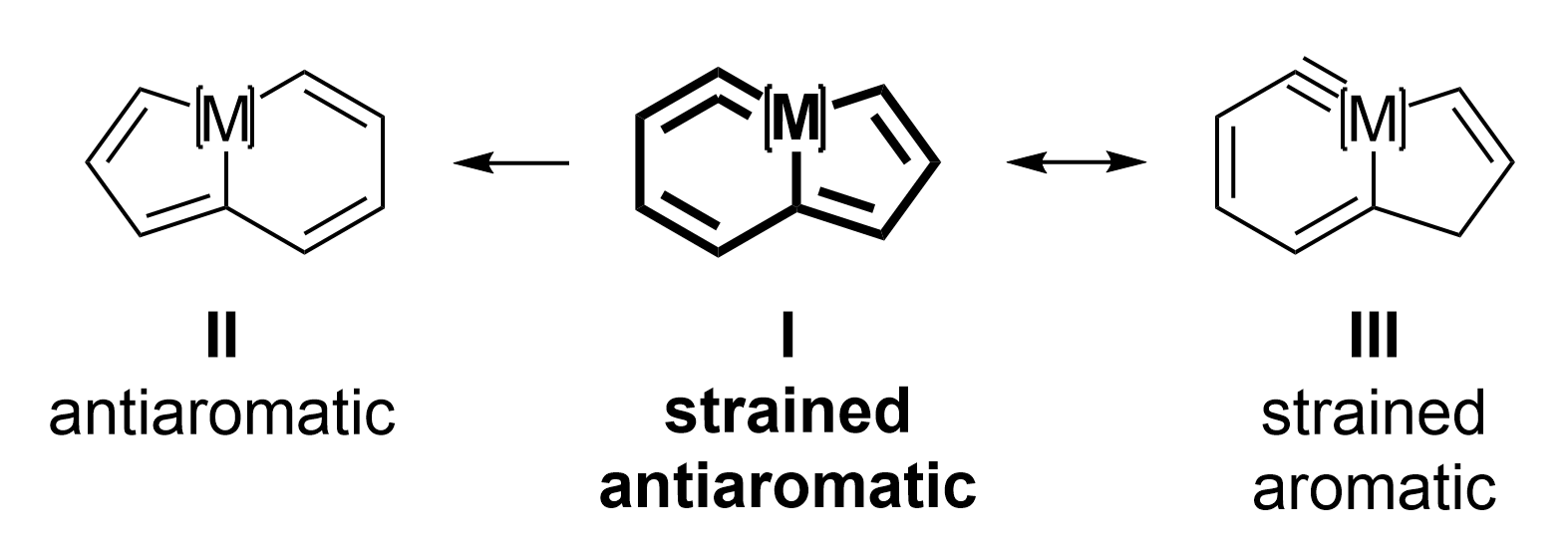
With this unusual metallacycle in hand, the team studied its reactivity. They observed a skeletal rearrangement of the strained antiaromatic framework assisted by nucleophiles to give another antiaromatic species II (pictured above). In addition, the team found acid/base-induced antiaromatic–aromatic switching between I and the aromatic species III (pictured above). Overall, the work advances the chemistry of strained and antiaromatic compounds and could be useful, e.g., for the development of switchable optoelectronic materials.
- Isolation, Reactivity, and Tunable Properties of a Strained Antiaromatic Osmacycle,
Qian Li, Yuhui Hua, Chun Tang, Dafa Chen, Ming Luo, Haiping Xia,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c00942