Species with multiple bonds between main-group atoms can be interesting research targets in inorganic chemistry. Multiple bonds between group 13 elements and group 15 elements, for example, pose a challenge for synthesis because the products tend to oligomerize. However, they could also undergo, e.g., cycloadditions with other species, forming heterocycles. Heterocycles such as borazines, which contain B and N atoms, are well known, but heterocycles containing P and Al are rare.
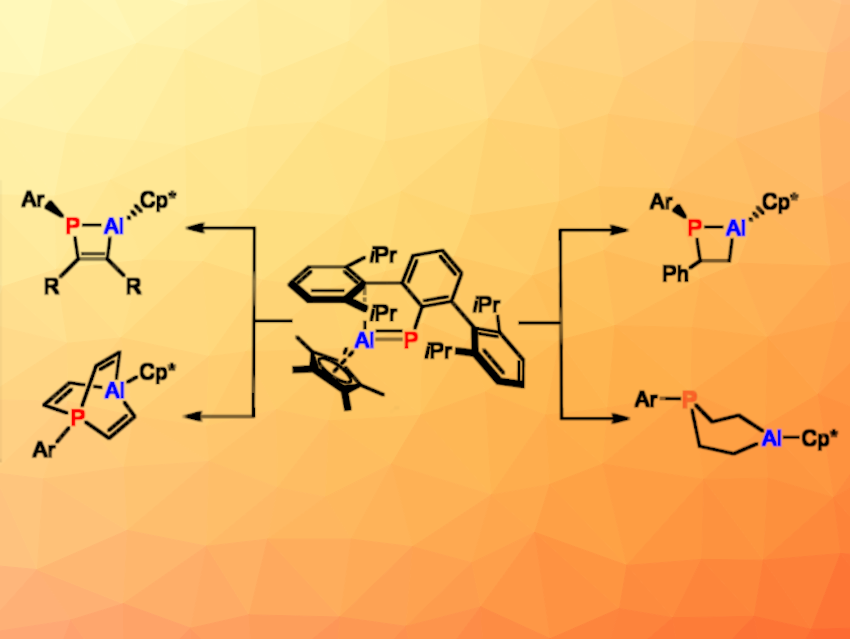
Milica Feldt, Christian Hering-Junghans, Leibniz-Institut für Katalyse e.V. (LIKAT), Rostock, Germany, Holger Braunschweig, University of Würzburg, Germany, and colleagues have reacted molecules with a double bond between P and Al, phosphaalumenes (example pictured above in the center) with alkenes and alkynes (example products pictured above). The team first synthesized a phosphaalumene by reacting TipTerP(PMe3) (TipTer = 2,4,6-(iPr3C6H2)-C6H3) with (Cp*Al)4 (Cp* = 1,2,3,4,5-pentamethylcyclopentadiene).
The resulting TipTerP=AlCp* and the previously known DipTerP=AlCp* (DipTer=2,6-Dip-C6H3) were then reacted with the alkynes PipC≡CPip (Pip = piperidyl) or PhC≡CPh, forming 1,2-phosphaalumetes (pictured above in the top left). When DipTerP=AlCp* was reacted with the sterically less demanding HC≡CH or HC≡CSiMe3, it instead formed 1,4-phosphaaluminabarrelenes (example pictured above in the bottom left).
When the team reacted the phosphaalumenes with alkenes such as styrene, they obtained saturated four-membered ring species (pictured above in the top right). A reaction of DipTerP=AlCp* with ethylene gave an 1,4-aluminaphosphorinane (pictured above on the bottom right). Overall, the team obtained a range of unprecedented P,Al-heterocycles.
- On the Reactivity of Phosphaalumenes towards C−C Multiple Bonds,
Samuel Nees, Tim Wellnitz, Fabian Dankert, Marcel Härterich, Simon Dotzauer, Milica Feldt, Holger Braunschweig, Christian Hering-Junghans,
Angew. Chem. Int. Ed. 2022.
https://doi.org/10.1002/anie.202215838