Enzymatic oxidation and reduction processes are often mediated by nicotinamide adenine dinucleotide (NAD). Its oxidized form NAD+ contains a pyridinium fragment that can formally bind and release a hydride unit. It can be isolated and used for chemical synthesis, but it is expensive as a stoichiometric cofactor. Synthetic analogues can, thus, be useful.
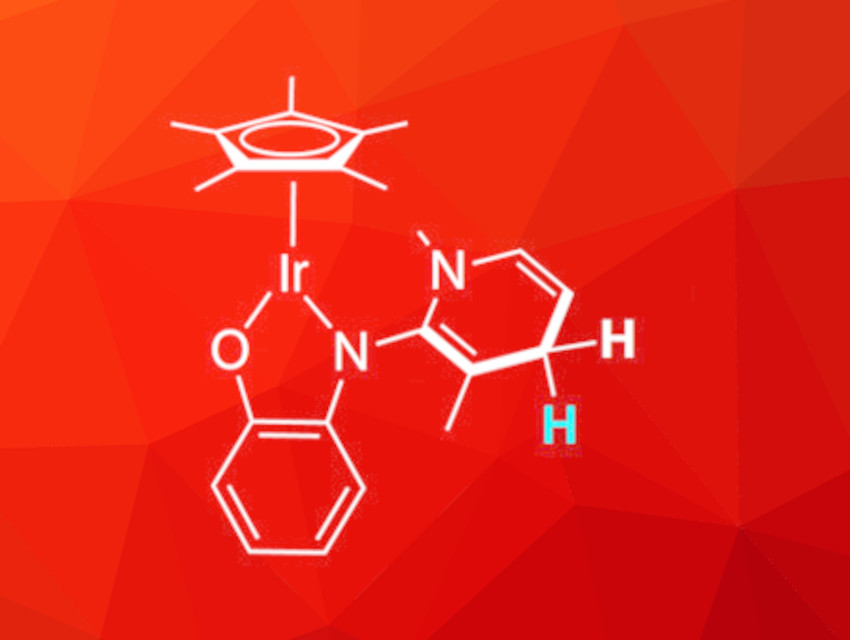
Martin Albrecht, University of Bern, Switzerland, and colleagues have developed an iridium complex with a pyridinium-based ligand, IrPYE+. This cationic complex can be hydrogenated using formates. This hydrogenation does not occur at the metal center but at the ligand, forming the neutral, dearomatized form of the pyridinium unit, analogous to the NAD+/NADH couple. This reduced complex (IrPYEH, pictured) is stable against air and moisture.
The complex was used for the reduction of aromatic aldehydes and ketones to the corresponding alcohols. The reaction was carried out in DMSO at 40 °C in the presence of triethylammonium formate. Quantitative conversion was achieved after 1.5 h with 0.1 mol% catalyst. When the reaction time was extended to 32 h, even catalyst loadings as low as 0.001 mol% achieved quantitative conversion.
Ketones were also reduced, but at a slightly slower rate. The catalyst also quantitatively reduced 4-carboxy-benzaldehyde and benzoic acid-contaminated benzaldehyde to the corresponding alcohols, which shows the acid tolerance of the system. When a deuterated formate was used, highly selective deuteration of the substrates was achieved. Thus, the developed easy-to-handle hydrogenation catalyst is useful for both hydrogenations and selective deuterations.
- NADH-Type Hydride Storage and Release on a Functional Ligand for Efficient and Selective Hydrogenation Catalysis,
Nicolas Lentz, Sabela Reuge, Martin Albrecht,
ACS Catal. 2023.
https://doi.org/10.1021/acscatal.3c02343