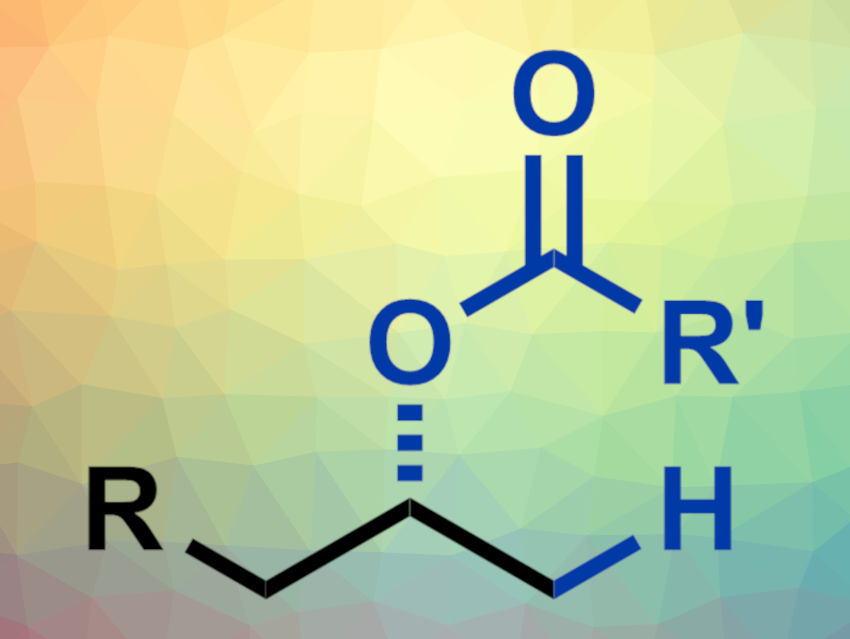
Enantiopure alcohols can be useful intermediates and products in organic synthesis. One possible way to synthesize them could be the use of hydrooxygenation reactions of alkenes to form a C–O bond. However, this type of reaction is challenging, and a catalytic asymmetric hydrooxygenation of alkenes with good enantioselectivity had not been reported so far.
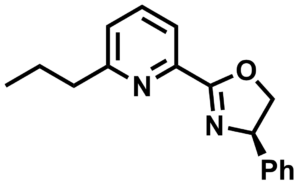
Pinhong Chen, Shanghai Institute of Organic Chemistry, University of Chinese Academy of Sciences, Guosheng Liu, Shanghai Institute of Organic Chemistry and East China Normal University, Shanghai, and colleagues have developed a palladium(II)-catalyzed enantioselective hydrooxygenation of unactivated terminal alkenes (example product pictured above) that uses a chiral substituted pyridinyl oxazoline (Pyox) ligand (pictured below).
The team reacted a wide variety of terminal alkenes with a range of different carboxylic acids using Pd(OAc)2 together with the Pyox ligand as a catalyst, (EtO)2MeSiH/benzoquinone (BQ) as a redox system, and Cl2CHCHCl2 (TCE) and water as solvents. They obtained optically pure esters in good yields and with excellent enantioselectivities. These ester products can be easily hydrolyzed to obtain chiral alcohols. The hydrooxygenation reaction has a broad substrate scope, including natural product and drug derivatives, and proceeds under mild conditions.
- Palladium(II)-Catalyzed Enantioselective Hydrooxygenation of Unactivated Terminal Alkenes,
Xintuo Yang, Xiang Li, Pinhong Chen, Guosheng Liu,
J. Am. Chem. Soc. 2022.
https://doi.org/10.1021/jacs.2c02753