Sulfones are useful products and intermediates, e.g., in organic synthesis, medicinal chemistry, and materials chemistry. SO2 insertion processes can be useful for their synthesis in a step-economic manner. However, this type of reaction often requires noble-metal catalysts. The development of methods that use Earth-abundant metals would, thus, be useful.
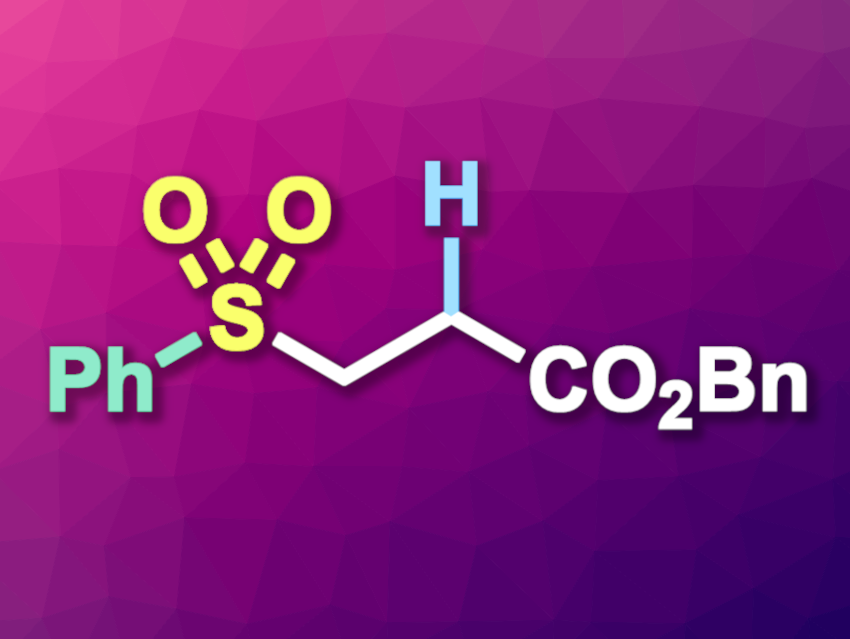
Jie Wu, Taizhou University, China, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, and Henan Normal University, Xinxiang, China, and colleagues have developed a nickel-catalyzed method for the hydrosulfonylation of alkenes via an SO2-insertion strategy using readily available aromatic iodides and K2S2O5 (example product pictured). The team reacted a variety of electron-deficient alkenes with a range of aromatic iodides and K2S2O5 in the presence of the organosilane (iPr)3SiH as the reductant, using NiCl2·(PPh3)2 as the catalyst precursor and 1,10-phenanthroline as a ligand. The reactions were performed in a mixture of tetrahydrofuran (THF) and dimethylformamide (DMF) at 70 °C.
The desired aryl alkyl sulfones were obtained in moderate to high yields. A model reaction at the 2-mmol scale gave the product in a yield of 66 %. According to the researchers, the reactions proceed via sulfinate intermediates, which undergo Michael-type additions to the alkenes. Overall, the method has a broad substrate scope, uses an inexpensive catalyst and simple starting materials, and it can be used for the late-stage modification of complex molecules.
- Ni-Catalyzed Hydrosulfonylation of Alkenes with Aromatic Iodides and K2S2O5,
Jun Zhang, Huiling Ma, Chenxi Yang, Jie Dang, Jiemin Xia, Jie Wu,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c03397