Nitrous oxide (N2O) is an environmentally harmful gas. It has a greenhouse effect and depletes ozone. Using N2O to produce value-added compounds is, thus, an interesting research target. It could, for example, serve as an oxygen-transfer reagent, leaving only nitrogen as a harmless byproduct.
Ruben Martin, Institute of Chemical Research of Catalonia (ICIQ), The Barcelona Institute of Science and Technology, Tarragona, Spain, and ICREA, Barcelona, Spain, and colleagues have developed a nickel-catalyzed C(sp2)–H hydroxylation of aryl bromides that uses N2O as an oxygen-atom source. The team reacted a wide variety of aryl bromides with a range of alkynes and N2O, using NiBr2·diglyme (diglyme = bis(2-methoxyethyl) ether) as the catalyst together with a phenanthroline-based ligand, Mn as a reductant, and NaI as an additive. The reactions were performed in dimethylacetamide (DMA) at 50 °C.
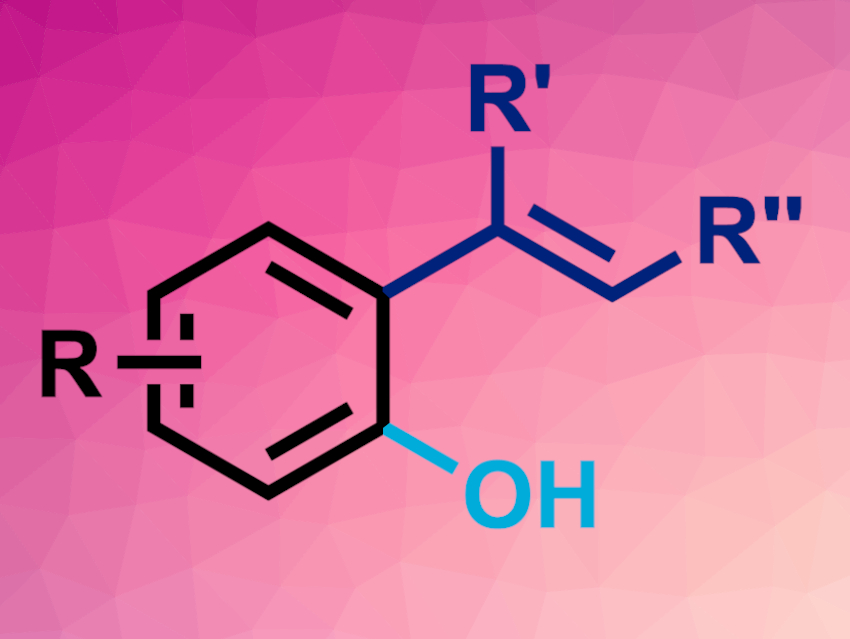
The team obtained ipso/ortho difunctionalized products (general structure pictured). They propose that the reaction involves a 1,4-Ni translocation, followed by N2O insertion. The products were obtained in moderate to good yields and with high regio- and stereoselectivities. When a different ligand is used, the selectivity of the process changes and the approach can also be used to give ketones rather than phenols. Such regiodivergent oxygen-transfer reactions could be a promising way to repurpose N2O.
- C(sp2)–H Hydroxylation via Catalytic 1,4-Ni Migration with N2O,
Huihui Zhang, Jesus Rodrigalvarez, Ruben Martin,
J. Am. Chem. Soc. 2023.
https://doi.org/10.1021/jacs.3c07018