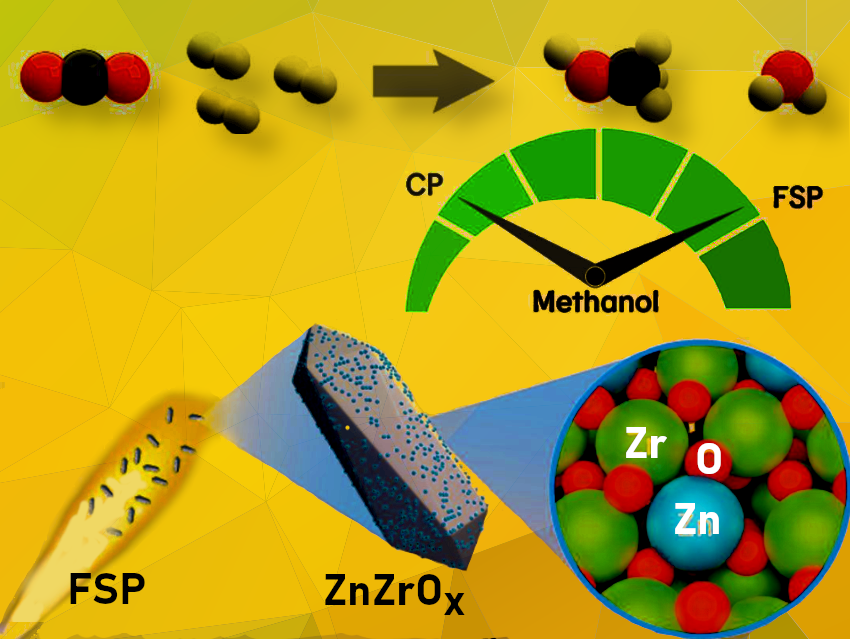
Methanol, a versatile energy carrier, currently has an annual production capacity of over 4 million metric tons. However, demand is expected to surpass production by 2023. While methanol is used in various applications, including fuel cells and combustion engines in the maritime shipping industry, its production relies on fossil feedstocks. Therefore, it is urgent to align methanol production with global efforts to reduce carbon footprint. One possibility is to use captured CO2 and renewable H2 to produce methanol through thermocatalytic conversion. Significant efforts have been made to identify promising catalytic materials for sustainable methanol production. Mixed zinc-zirconium oxides, ZnZrOx, are highly selective and stable catalysts for CO2 hydrogenation to methanol. However, their activity remains moderate, and there is a lack of descriptors to design improved systems.
To address this issue, Javier Pérez-Ramírez, ETH Zurich, Switzerland, and colleagues have applied flame spray pyrolysis (FSP), a one-step and scalable method, to prepare and systematically investigate a platform of ZnZrOx catalysts with a broad range of compositions (0–100 mol% Zn) and compared them to state-of-the-art coprecipitated (CP) analogs.
FSP offers effective control over the nanostructure of the synthesized materials, favoring the surface deposition of active metal species on carriers without promoting bulk incorporation, a classic drawback of CP methods.
The researchers found that FSP systems with Zn contents up to 5 mol% displayed a threefold higher methanol productivity compared to their CP counterparts. In-depth characterization and theoretical simulations showed that FSP maximizes the surface area and formation of atomically dispersed Zn2+ sites incorporated in lattice positions within the ZrO2 surface, which is key to improving performance. In situ electron paramagnetic resonance (EPR) spectroscopy analysis revealed that the specific architecture of the flame-made catalyst markedly fosters the generation of oxygen vacancies. Together with surrounding Zn and Zr-O atoms, the oxygen vacancies create active ensembles that favor methanol formation through the formate path. At the same time, they suppress undesired CO production, as confirmed by kinetic modeling.
The study offers promising design and practice guidelines for the use of ZnZrOx catalyst systems in methanol production from CO2, and according to the scientists, this low-cost and earth-abundant catalyst family is driving ZnZrOx-catalyzed methanol synthesis.
- Design of Flame-Made ZnZrOx Catalysts for Sustainable Methanol Synthesis from CO2,
Thaylan Pinheiro Araújo, Jordi Morales-Vidal, Tangsheng Zou, Mikhail Agrachev, Simon Verstraeten, Patrik O. Willi, Robert N. Grass, Gunnar Jeschke, Sharon Mitchell, Núria López, Javier Pérez-Ramírez,
Advanced Energy Materials 2023.
https://doi.org/10.1002/aenm.202204122