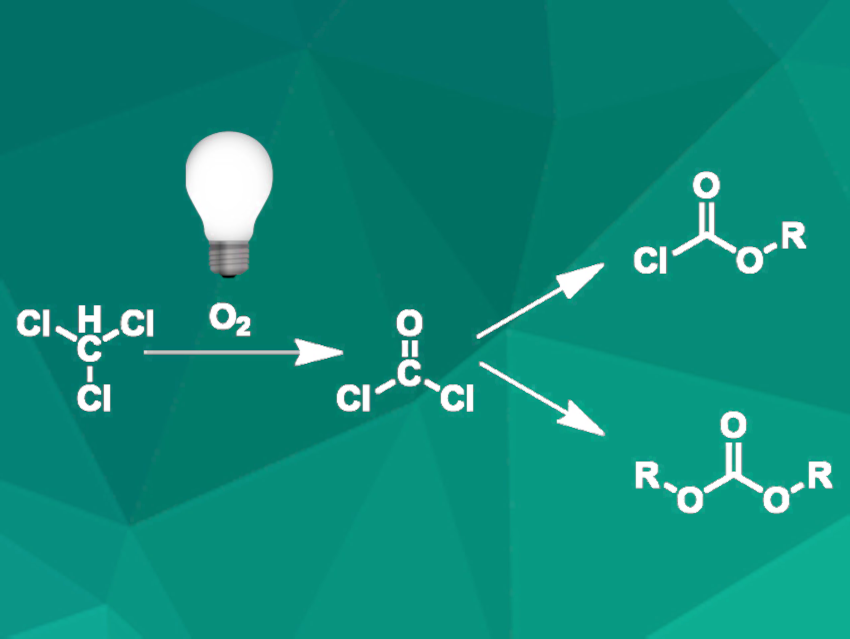
Phosgene is an important intermediate in chemical manufacturing processes, e.g., in the synthesis of polycarbonate polymers or some pesticides. Due to its high toxicity, the storage and handling of large quantities of phosgene are problematic. This issue can be tackled by using in-situ processes where phosgene is not stored but only formed in the required amount and directly converted to the desired product.
Akihiko Tsuda, Kobe University, Japan, and colleagues have developed a process for the photochemical oxidation of chloroform to phosgene in continuous flow. They used a quartz-glass tube reactor with a mercury lamp as a source of UV radiation. Chloroform was pre-mixed with oxygen and injected into the photoreactor. The quantity of phosgene formed was measured by a subsequent reaction with 1-butanol, which yielded butyl chloroformate and dibutyl carbonate (pictured).
Under optimized operating conditions, a phosgene yield of 96 % was achieved. The researchers suggest that the reaction proceeds via a photoinduced radical chain reaction which also yields some hexachloroethane. This byproduct can lead to clogging of the reactor, but its formation was successfully prevented by increasing the oxygen flow rate.
Semi-Batch and Flow Syntheses
The photochemical reactor was then used for the semi-batch synthesis of chloroformates and carbonate esters with pyridine as an added base. At room temperature, a mixture of these two products was formed, but heating with excess alcohol to 50 °C led to quantitative conversion to the carbonates. This reaction worked for different alcohols, including bisphenols which give polycarbonates.
Additionally, a continuous-flow system was established for this reaction. With this approach, alkyl alcohols reacted to the chloroformates in good yields, but no product was observed for fluorinated alcohols. This was remedied by the addition of N-methylimidazole. Under these modified conditions, fluorinated alcohols gave carbonates in up to 60 % yield.
- Flow Photo-On-Demand Phosgenation Reactions with Chloroform,
Yue Liu, Itsuumi Okada, Akihiko Tsuda,
Org. Process Res. Dev. 2022.
https://doi.org/10.1021/acs.oprd.2c00322