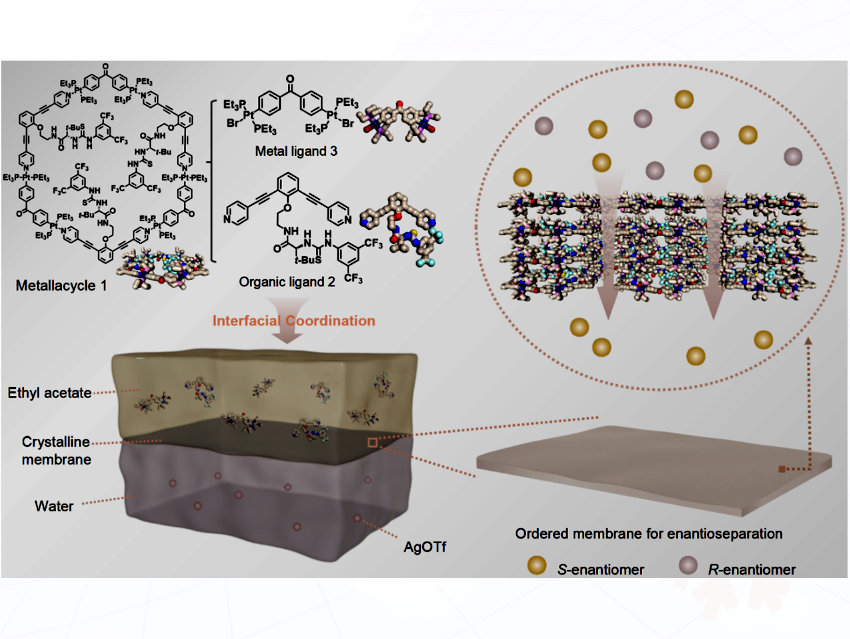
The separation of chiral molecules—mirror-image compounds critical to drug safety and efficacy—is of significant importance for pharmaceutical manufacturing. While membrane technology offers an energy-efficient alternative to traditional chromatography, existing designs face challenges in achieving simultaneously high selectivity, fast permeation, and scalable fabrication. Crucially, most chiral membranes lack sufficient ordered pore structures for precise enantioseparation.
Yue Sun from Tiangong University, China <bold>, and co-workers have proposed a strategy using interfacial coordination-driven self-assembly. They engineered a crystalline metallacyclic membrane (CMM) by reacting chiral thiourea ligands and metal ligands at a water/ethyl acetate interface. The resulting ultrathin, defect-free membrane features intrinsically chiral, ordered pores that act as molecular sieves.
When supported on polyacrylonitrile (PAN), the CMM-PAN composite membrane achieves unprecedented performance in separating racemic mixtures: High permeation flux (4.9 mmol m⁻2> h⁻1) and 100% enantiomeric excess selectivity for 1-phenylethanol, 1-phenylethylamine, and 2-phenylglycinol. Thermodynamic data (ΔH<italics>*, TΔS<italics>*, ΔG<italics>*) and molecular simulations revealed the retarded-transport mechanism: R-enantiomers preferentially adsorb to chiral thiourea sites within the membrane, slowing their transport, while permitting S-enantiomers to permeate efficiently. To demonstrate industrial potential, the researchers integrated the membrane into a 3D-printed crossflow module, increasing flux 7.3-fold while maintaining high selectivity. Additionally, the membrane achieved highly efficient enantioseparation for the high-value pharmaceutical salbutamol.
This approach provides a feasible strategy for creating supramolecular metallacyclic channels in chiral membranes, demonstrating the potential for accurate enantioseparation.
- Interfacial Coordination Induced Crystalline Metallacyclic Membrane for High-Performance Enantioseparation,
Run-Hao Li, Yumei Wang, Yi Liu, Yue Sun,
Aggregate 2025.
https://doi.org/10.1002/agt2.70095