Polycyclic imidazoles are often found, e.g., in biologically active molecules. They can be prepared, for example, using transition-metal-catalyzed C–H cyclizations of imidazoles with alkenes. This approach provides a one-step route with good atom economy. However, it is often limited to exo-selective cyclizations. Corresponding endo-selective cyclizations have been more difficult to achieve.
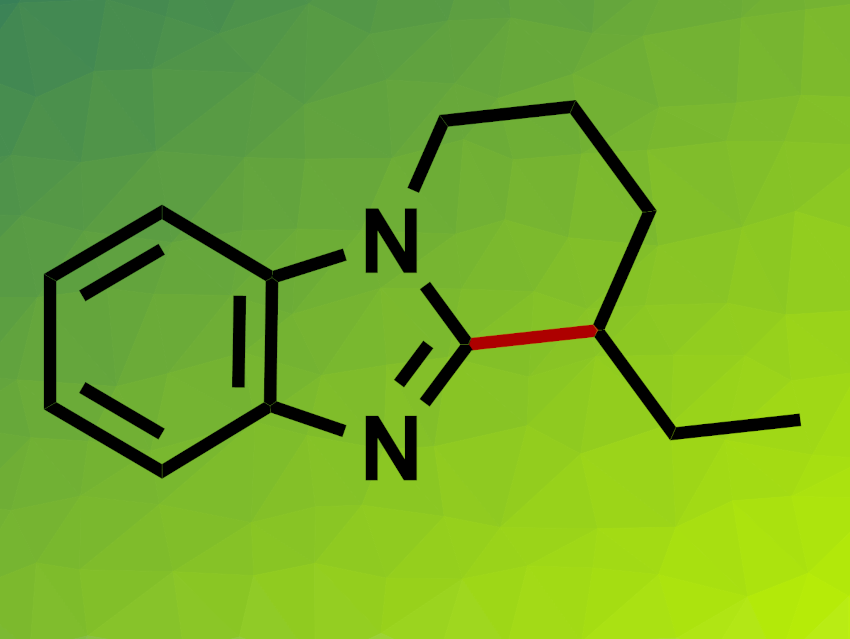
Xue-Tao Xu, Wuyi University, Jiangmen, China, Mengchun Ye, Wuyi University and Nankai University, Tianjin, China, and colleagues have developed a nickel-catalyzed intramolecular endo-selective C–H cyclization of benzimidazoles with alkenes (example product pictured). The team used Ni(cod)2 (cod = 1,5-cyclooctadiene) as a catalyst together with an N-heterocyclic carbene (NHC) ligand precursor and KOtBu as a base, as well as AlMe3. The reactions were performed in toluene at 70 °C.
Under these conditions, different benzimidazole derivatives functionalized with internal and terminal alkenes were converted to the desired cyclization products with high yields and high endo-selectivity. (Z)-alkenes gave a lower yield and lower endo-selectivity than (E)-alkenes. Substrates with electron-withdrawing substituents on the phenyl ring were not well suited to the reaction. According to the researchers, the endo-selectivity can be attributed to steric repulsion in a reaction intermediate that involves both Ni and Al. Thus, the Ni–Al bimetallic catalyst plays an important role in the selectivity of the transformation.
- Catalyst-Controlled Nickel-Catalyzed Intramolecular endo-Selective C–H Cyclization of Benzimidazoles with Alkenes,
Zi-Jian Liu, Jiang-Fei Li, Feng-Ping Zhang, Xue-Tao Xu, Mengchun Ye,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.2c04012
Update (January 8, 2023)
The article originally had AlCl3 in place of AlMe3; this has been corrected.



![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)