Some enzymes can be used in chemical synthesis, and their activity can often be expanded beyond the limits of native reactions using non-native substrates, reagents, and reaction conditions. Enzymes that depend on vitamin B12 are interesting in this context, because the B12 cofactor can participate in catalytic cycles involving its Co(I)-(III) oxidation states. However, using this approach in chemical synthesis requires control over the reactivity and selectivity of the reaction.
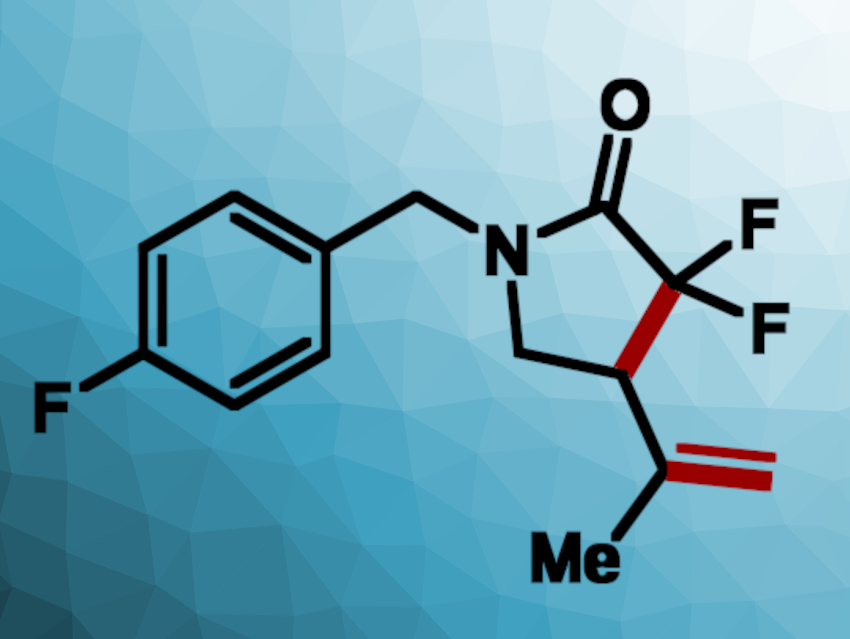
Jared C. Lewis, Indiana University, Bloomington, USA, and colleagues have repurposed the B12-dependent transcription factor CarH for non-native radical cyclization reactions. The team prepared an engineered variant of the enzyme, called CarH*, which can catalyze the radical cyclization of chlorodifluoroacetamides with alkene substituents to give difluorinated lactams (example product pictured above). The products have multiple useful functional groups and can be further transformed.
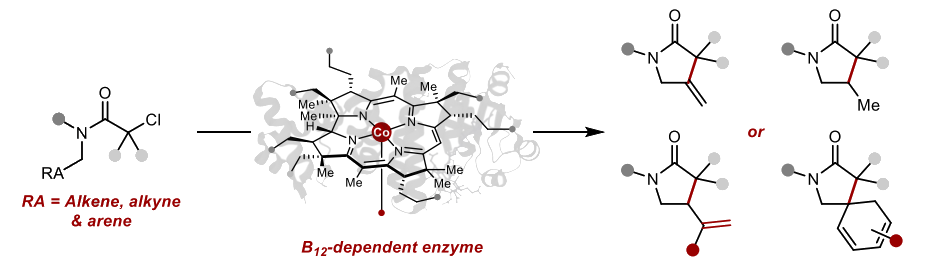
The modified enzyme CarH* also catalyzes the reductive dearomative spirocyclization of substrates with aromatic substituents, giving spirobicyclic products with an uncommon 1,3-cyclohexadiene unit (schematically pictured above, bottom right).
B12 alone can also catalyze similar reactions to some extent. The work shows that the protein scaffolds of B12-dependent enzymes can be used to control the reactivity of B12, provide improved yields and selectivities compared with B12 alone, and expand the catalytic toolbox.
- Non‐native Intramolecular Radical Cyclization Catalyzed by a B12‐Dependent Enzyme,
Jianbin Li, Amardeep Kumar, Jared C. Lewis,
Angew. Chem. Int. Ed. 2023.
https://doi.org/10.1002/anie.202312893