Organic luminescent radicals, with their unique open-shell electronic structures, hold great promise for optoelectronic applications. However, their intrinsic luminescence in aggregated states has been difficult to achieve due to severe aggregation-caused quenching (ACQ) from strong intermolecular interactions.
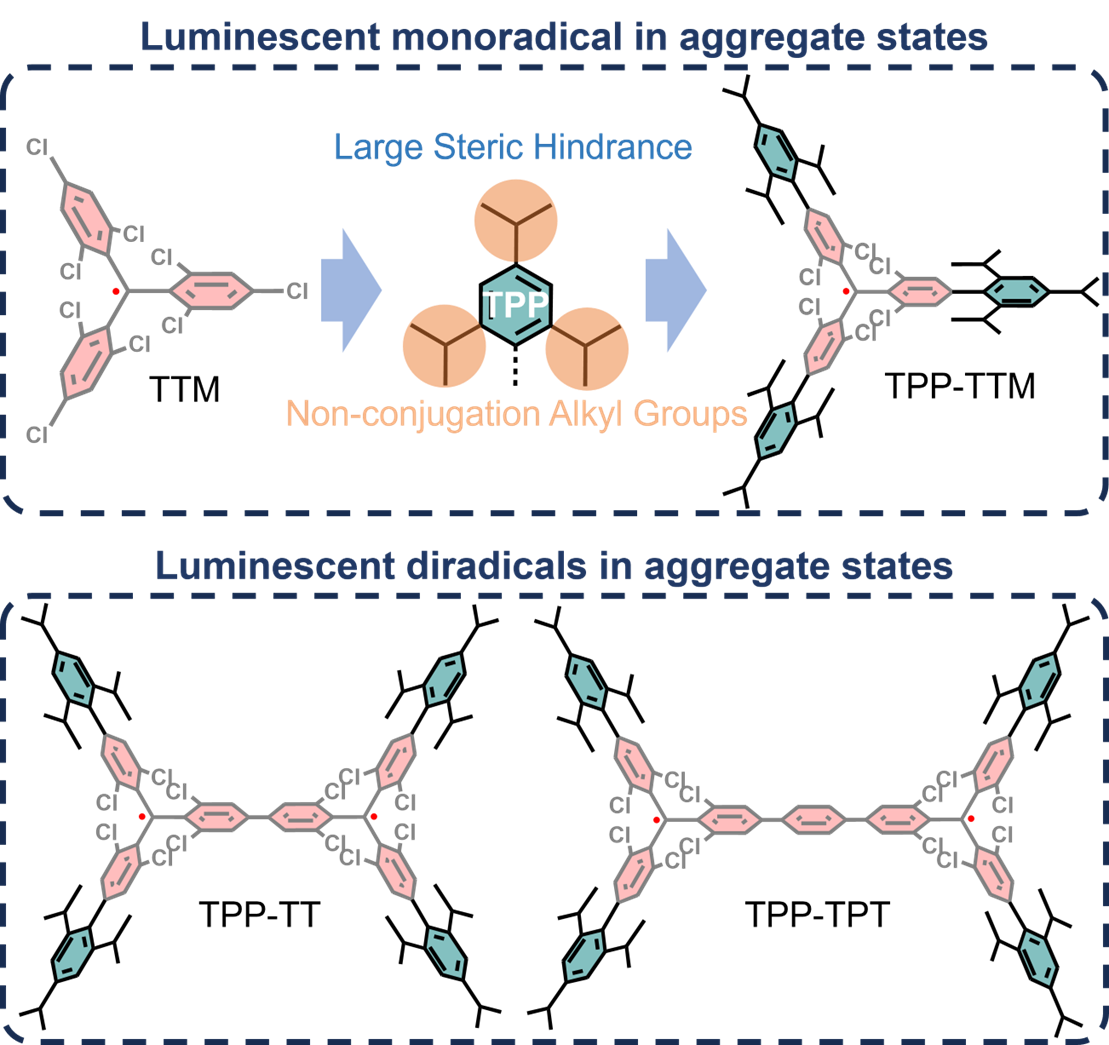
A research team comprising Xin Ai (Hainan University, China), Alim Abdurahman (Jilin University, China), and Qiming Peng (Nanjing Tech University, China) proposed a molecular design strategy featuring the introduction of triphenylphosphine (TPP) groups onto the classical triphenylmethyl (TTM) radical. This approach effectively increases steric hindrance and interrupts intermolecular conjugation, suppressing detrimental aggregation effects.
Using this strategy, the team synthesized TPP-TTM radicals, which exhibited bright intrinsic luminescence across various aggregated states—including crystals, powders, and amorphous films—achieving, for the first time, pure radical emission in these states without the need for doping or external matrices. Single-crystal X-ray diffraction and theoretical calculations revealed that the bulky TPP substituents effectively reduce intermolecular interactions, thereby suppressing ACQ. Furthermore, this molecular design strategy was successfully applied to classical diradicals such as Chichibabin’s and Müller’s hydrocarbons, demonstrating its broad applicability.
This work enriches molecular design strategies for luminescent radicals and opens new avenues for their application in solid-state luminescence, advancing the development of next-generation organic light-emitting materials.
- Achieving Intrinsic Luminescence of Pure Organic Mono- and Di-Radicals in Aggregated States
Jiahao Guan, Zihao Zhu, Quanquan Gou, Jingmin Wang, Zhiyuan Kuang, Lintao Zhang, Xuewei Zhang, Xin Ai*, Alim Abdurahman*, and Qiming Peng*,
Aggregate 2025
https://doi.org/10.1002/agt2.70100