Crystalline porous materials, including zeolites, metal–organic frameworks (MOFs), and covalent organic frameworks (COFs), have attracted significant interest owing to their well-ordered pore structures, high surface areas, and tunable functional properties. These attributes make them promising candidates for applications such as gas storage and catalysis. Nevertheless, their practical use is often hampered by poor solution processability, as they typically exist as insoluble crystalline powders.
In contrast, molecular-based porous materials—such as macrocycles, molecular cages, and hydrogen-bonded organic frameworks—show excellent solution processability and can be readily recycled via recrystallization. These discrete systems assemble into porous networks through weak intermolecular interactions. However, they frequently suffer from structural instability and difficulties in maintaining permanent porosity. Furthermore, optimizing their performance requires precise structural engineering, tailored functionalization, and computationally guided design, all of which introduce considerable synthetic and conceptual challenges.
Ion–dipole interactions constitute an important category of non-covalent forces that occur between ions and polar molecules or molecular dipoles. These electrostatic interactions arise when the electric field of an ion aligns nearby polar groups, resulting in attraction. In terms of strength, ion–dipole interactions generally lie between hydrogen bonds and coordination bonds, with energies varying by system; for instance, crown ether–alkali metal complexes can eshow bond energies of 100–150 kJ/mol. This intermediate strength offers a balance between stability and reversibility, making ion–dipole interactions particularly attractive for designing materials that are both structurally robust and capable of undergoing reversible assembly under mild conditions.
Although these interactions have shown promise in tetravalent borate chemistry for improving long-range crystallinity and local flexibility, their broader application remains limited due to high solvation effects and a lack of directionality.
Ming Liu, Zhejiang University, China, and colleagues have reported an innovative design strategy that exploits the unique coordination properties of R[SiO₄]⁻ clusters to develop a new class of amphibious crystalline porous materials stabilized by ion–dipole interactions. The designed silicate building blocks incorporate ethylene glycol–like coordination motifs, in which the silicon center further enhances the Lewis basicity of electron-rich oxygen atoms relative to conventional [BO₄]⁻ building blocks. This creates exceptionally selective hard basic sites with strong binding affinity toward alkali metal ions.
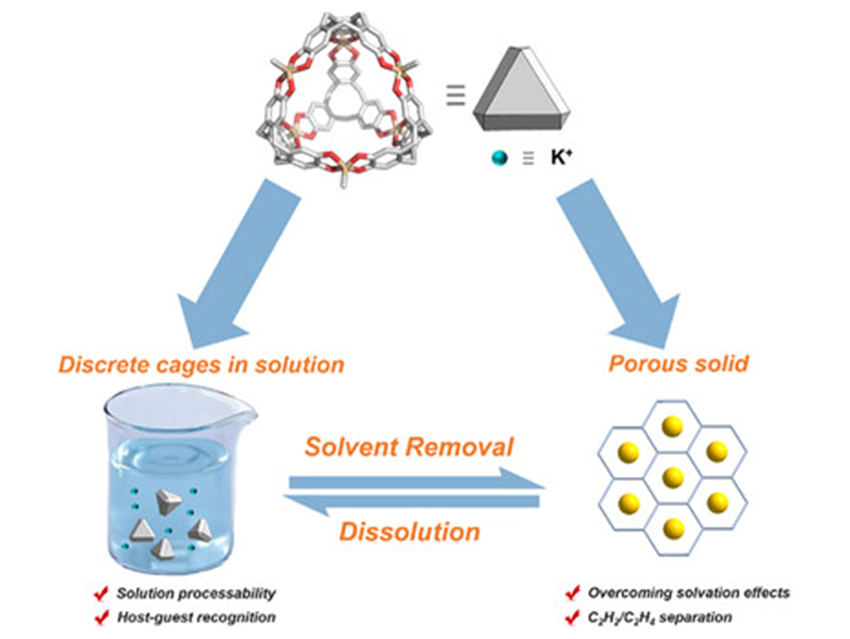
This distinctive molecular design successfully addresses the critical challenge of alkali metal ion solvation: the dense aggregation of ion–dipole interactions between silicate units and metal ions provides sufficient binding energy to overcome the solvation energy barrier. Building on this principle, the researchers constructed two crystalline porous materials with distinct dia and hcb topologies from silicate cages. Single-crystal X-ray diffraction reveals their three-dimensional channel structures and the precise coordination geometries between silicate units and alkali metal ions.
Importantly, these materials retain the excellent solution processability characteristic of molecular porous systems, enabling complete regeneration. They uniquely combine the intrinsic host–guest recognition of molecular cages with permanent porosity and gas adsorption capabilities in the solid state.
This work systematically demonstrates the use of ion–dipole interactions to construct porous materials that integrate high structural stability with excellent processability, directly addressing a longstanding challenge in the field. Moreover, facile regeneration via solvent removal and recrystallization enhances their appeal for potential industrial applications.
- Ion-Dipole Interaction-Driven Assembly of Silicate Cages
Yutao Guan, Hongqing Li, Ju Yang, Dingyue Hu, Saisai Yu, Ming Liu
Aggregate 2025
https://doi.org/10.1002/agt2.70157