A superatom is a cluster of atoms that mimics some of the behaviors of a single atom. Nanosized, chemically modified gold superatoms, for example, can be connected and used to assemble larger structures. The assembly of such gold superatoms can be achieved, e.g., by ligand exchange reactions with multidentate ligands. However, this type of reaction is difficult to control due to the large number of possible binding sites. Introducing open sites for binding at the superatoms could solve this issue by providing well-defined connection points, but still requires methods to control the formation of the exposed sites.
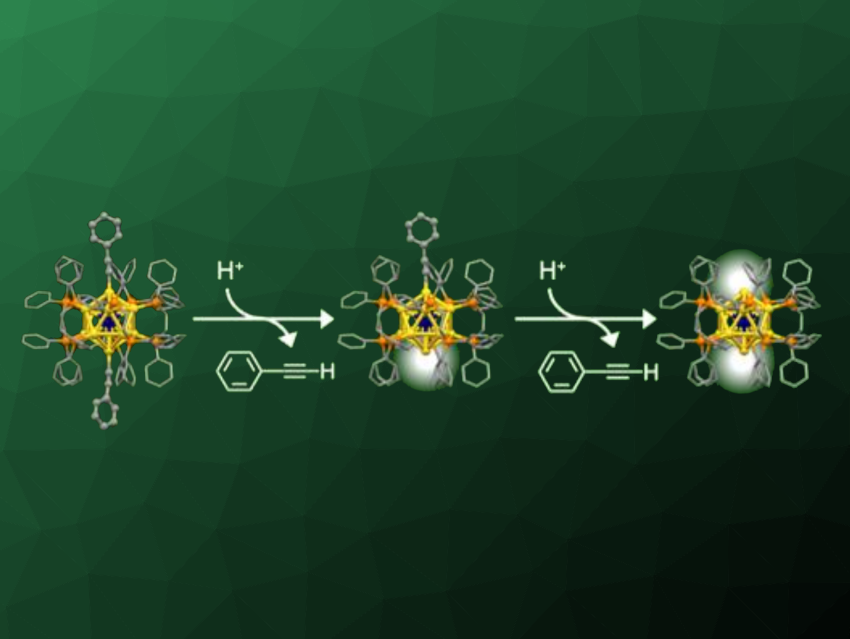
Tatsuya Tsukuda, The University of Tokyo, Japan, and colleagues have developed a method for the selective removal of anionic ligands from chemically modified superatoms. The team used a superatom of the type [IrAu12(dppe)5(PA)2]+ (dppe = 1,2-bis(diphenylphosphino)ethane, PA = phenylacetylide), which has two anionic PA ligands at the opposite sides of an icosahedral Ir@Au12 core. They successfully removed either one or two phenylacetylide ligands from this superatom by reactions with controlled amounts of tetrafluoroboric acid (pictured), obtaining [IrAu12(dppe)5(PA)]2+ or [IrAu12(dppe)5]3+, respectively. Apart from the targeted ligand removal, the structure of the cluster remained intact.
The team found that a single isocyanide molecule was selectively coordinated to the exposed site of [IrAu12(dppe)5(PA)]2+. They used this reactivity to assemble the superatoms into dimers and trimers. The researchers connected multiple clusters with either one or two open sites using 4,4”-p-terphenyldiisocyanide as a linear linker. This way, the assembly can be precisely controlled.
- Diphosphine‐Protected IrAu12 Superatom with Open Site(s): Synthesis and Programmed Stepwise Assembly,
Yuto Fukumoto, Tsubasa Omoda, Haru Hirai, Shinjiro Takano, Koji Harano, Tatsuya Tsukuda,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202402025