Ditetrylynes, i.e., the heavier group 14 analogues of alkynes (REER, E = Si, Ge, Sn, Pb), are interesting species. In contrast to the linear, triple-bonded structure of alkynes, they usually have a trans-bent structure and a lower bond order. One-coordinate free tetrylyne radicals of the type RE have been proposed as intermediates in the formation of ditetrylynes. While one-coordinate Sn(I) radicals have been found, the corresponding free Pb(I) radicals have remained elusive.
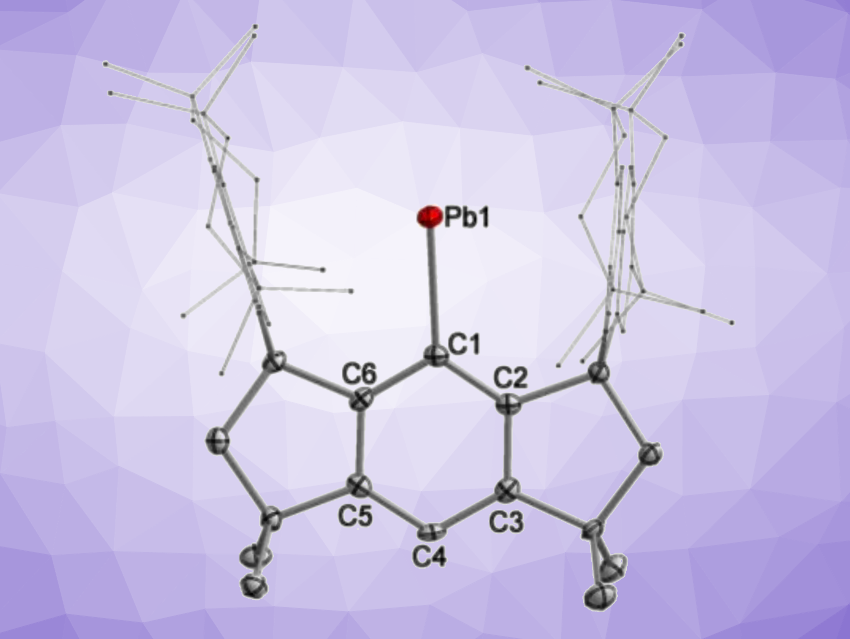
Shengfa Ye, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Gengwen Tan, Sun Yat-sen University, Guangzhou, China, and Soochow University, Suzhou, China, and colleagues have synthesized the first isolable free plumbylyne radical bearing a one-coordinate Pb(I) atom (simplified structure pictured). The team used a sterically demanding hydrindacene-based ligand to stabilize this species. They first prepared the corresponding plumbylene bromide precursor from a ligand-derived lithium salt and PbBr2. A debromination with potassium graphite (KC8) in tetrahydrofuran (THF) then gave the desired Pb(I) radical in a yield of 48 % as reddish brown crystals. The team found that the radical can coordinate an N-heterocyclic carbene (1,3,4,5-tetramethyl-imidazole-2-ylidene) to give the corresponding adduct, a two-coordinate Pb(I) radical, in a yield of 66 % as purple crystals.
The free Pb(I) radical was characterized using single-crystal X-ray crystallography, which confirmed that the Pb atom is one-coordinate and connected to the supporting ligand through a Pb–C bond with a length of 2.314 Å, corresponding to a single bond. There was no intermolecular Pb–Pb bonding interaction found. According to the researchers, the one-coordinate radical and its NHC adduct represent the first isolable examples of Pb(I) radicals.
- An Isolable One‐coordinate Lead(I) Radical with Strong g‐Factor Anisotropy,
Haonan Chen, Wang Chen, Dongmin Wang, Yizhen Chen, Zheng Liu, Shengfa Ye, Gengwen Tan, Song Gao,
Angew. Chem. Int. Ed. 2024.
https://doi.org/10.1002/anie.202402093