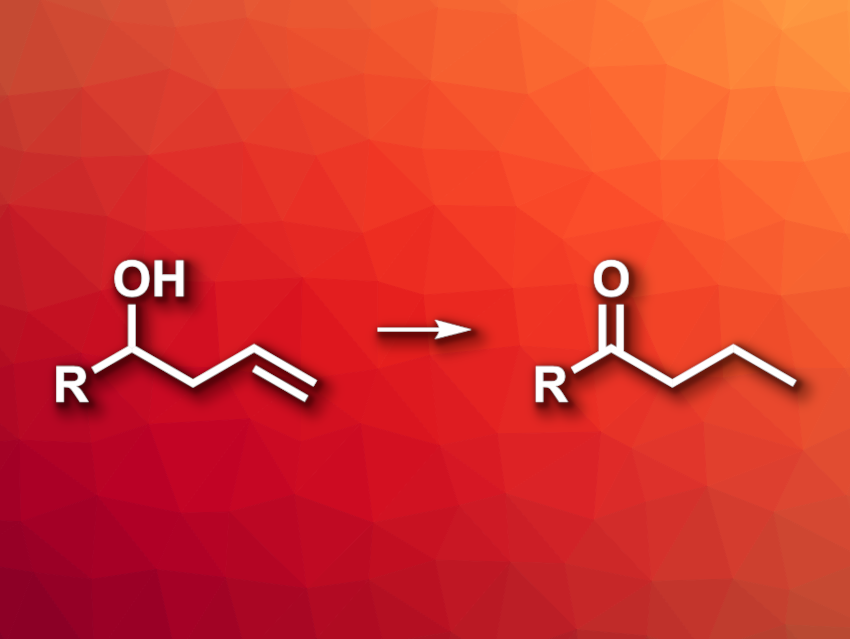
Transition-metal-catalyzed alkene isomerizations can be useful, atom-economical tools in organic synthesis. Isomerizations of allylic alcohols, for example, can be used for the synthesis of carbonyl compounds. Analogous reactions with more remote C=C bonds, e.g., using homoallylic alcohols, are less well explored, especially those using catalysts based on Earth-abundant metals.
Levi M. Stanley and colleagues, Iowa State University, Ames, USA, have developed a method for the nickel-catalyzed isomerization of homoallylic alcohols to give ketones (general reaction pictured). The team used a simple nickel catalyst, bis(cyclooctadiene)nickel(0) or Ni(COD)2, without a need for additional ligands and CsF as a base in the presence of cyclohexene. The reactions were performed in a dioxane solvent at 80 °C.
The desired ketones were obtained in yields of up to 98 %. The team converted a variety of α-aryl-substituted homoallylic alcohols, and the reaction also tolerates alkyl homoallylic alcohols. An isomerization of bishomoallylic alcohol was also successful when the reaction was performed with a higher loading of Ni(COD)2 and 2-propanol as an external hydride source. According to the researchers, the work addresses the limitations of previously reported iron catalysts, e.g., high catalyst loadings and a limited substrate scope.
- Nickel-Catalyzed Isomerization of Homoallylic Alcohols,
Dustin D. Youmans, Hai N. Tran, Levi M. Stanley,
Org. Lett. 2023.
https://doi.org/10.1021/acs.orglett.3c01201