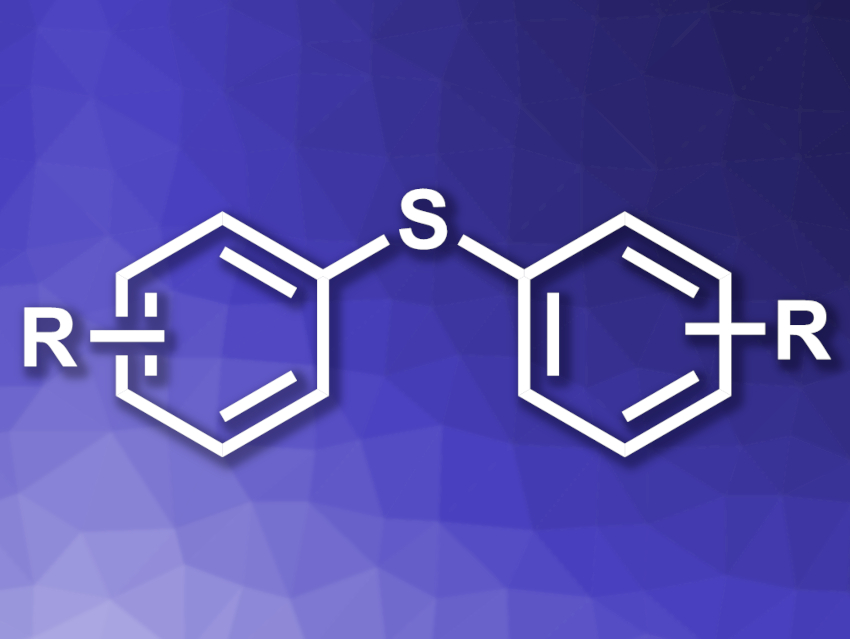
Sulfur-containing compounds are important in both Nature and organic synthesis. Aryl sulfides, for example, can be used as electrophiles or in photocatalysis. Existing methods for their synthesis often suffer from drawbacks such as the formation of byproducts with multiple sulfur atoms, a need for smelly sulfur reagents, or additional steps to reduce groups such as sulfoxides to sulfides.
Hideyuki Konishi, Kei Manabe, University of Shizuoka, Japan, and colleagues have developed a practical approach to synthesizing symmetrical diaryl sulfides (pictured) using iodoarenes and potassium metabisulfite (K2S2O5). K2S2O5 is usually employed as a sulfur dioxide (SO2) source, but in this case, it is used to introduce a divalent sulfur atom. The team reacted a variety of iodoarenes with K2S2O5 using Pd(OAc)2 as a catalyst, P(tBu)3·HBF4 as a ligand, NBu3 as an amine, and dimethylacetamide (DMA) as the solvent. The reactions were performed at 100 °C.
The desired symmetrical diaryl sulfides were obtained in generally moderate to high yields. The reaction tolerates a variety of substituents at the aryl component, even some bulky groups, as well as heterocyclic substrates. According to the researchers, the work could expand the utility of K2S2O5 as a sulfur source.
- Synthesis of Symmetrical Sulfides Enabled by a Sulfur Dioxide Surrogate Acting as a Divalent Sulfur Source,
Hideyuki Konishi, Ririka Fujita, Miyuki Yamaguchi, Kei Manabe,
Org. Lett. 2022.
https://doi.org/10.1021/acs.orglett.2c01284