Porous materials with tailored micropores can be very useful for separation processes. However, for compounds with almost identical molecular sizes and physical properties, it is challenging to engineer the pore structure of the adsorbent to achieve precise discrimination. One example for this is the kinetic sieving of CO2 from acetylene (C2H2), which have almost identical kinetic diameters.
Huabin Xing, Xili Cui, Zhejiang University, Hangzhou, China, and colleagues have synthesized sulfonic anion-based pillared ultramicroporous materials for the separation of C2H2 from CO2. They first synthesized the material ZU-610 from Cu2+, isonicotinic acid, 1,2-ethanedisulfonic acid (EDS), and a small amount of water. In the product, water oxygen atoms form Cu4(μ3-O)2 clusters with copper centers. These metal clusters are bound to the sulfonate units, forming one-dimensional chains that are connected by isonicotinic acid. This material shows a preferential affinity for C2H2 over CO2.
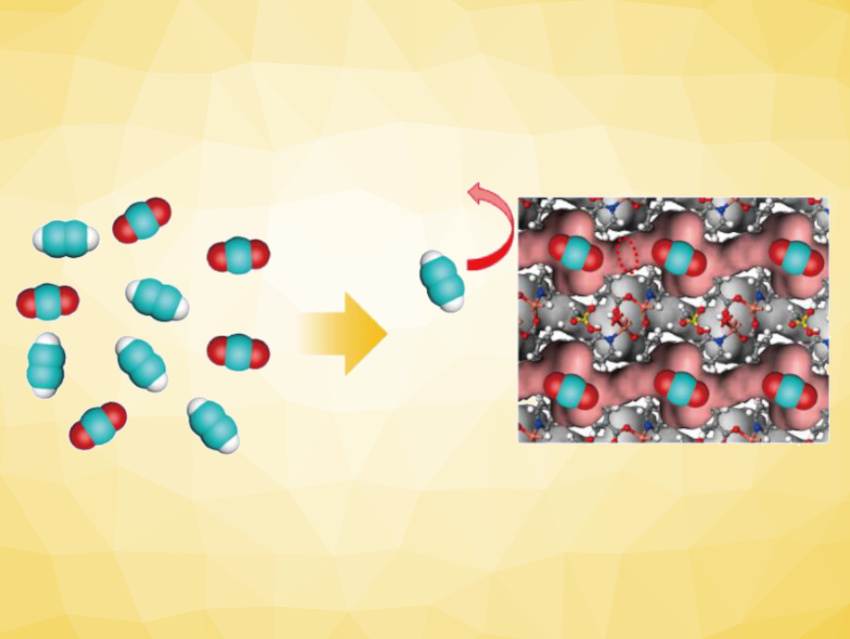
Then, the researchers prepared a modified material called ZU-610a with smaller pores by a post-synthetic heat treatment of ZU-610. The C2H2/CO2 selectivity was reversed in ZU-610a with its smaller pores, leading to CO2 selectivity. Using ZU-610a for kinetic sieving (pictured schematically), high-purity C2H2 (>99.5 %) was produced directly from binary mixtures of the two gases. Thus, tuning the pore structure has a significant influence on adsorption. The work could inspire new strategies in the development of adsorbent separation systems that can distinguish similar molecules.
- Kinetic‐Sieving of Carbon Dioxide from Acetylene through a Novel Sulfonic Ultramicroporous Material,
Jiyu Cui, Zhensong Qiu, Lifeng Yang, Zhaoqiang Zhang, Xili Cui, Huabin Xing,
Angew. Chem. Int. Ed. 2022.
https://doi.org/10.1002/anie.202208756