The greenhouse gas carbon dioxide could also serve as a chemical feedstock. However, its conversion into useful chemical compounds generally requires suitable catalysts due to the relative inertness of CO2. For example, lactonization and lactamization reactions involving aryl C–H bonds and CO2 that are catalyzed by transition metals are somewhat rare and tend to require catalysts based on precious metals.
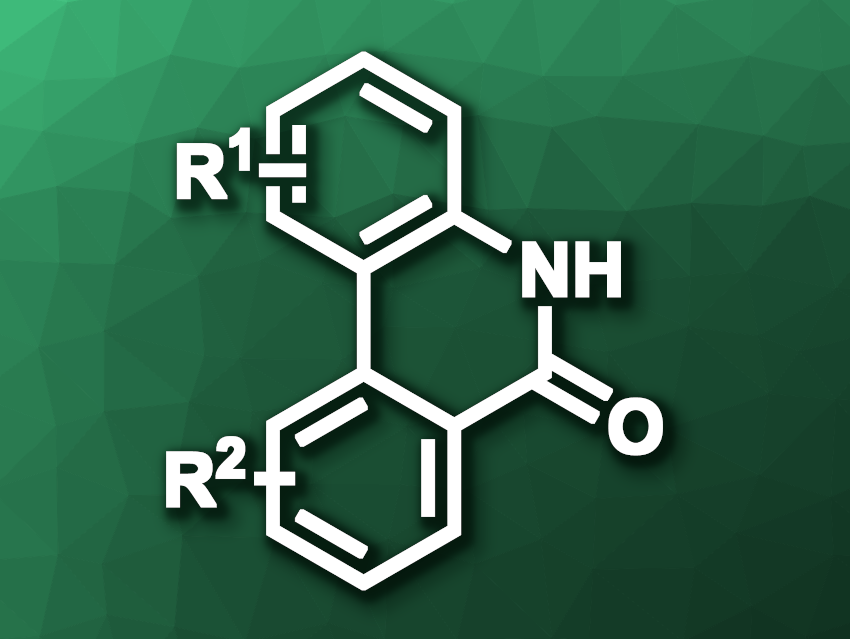
Baiquan Wang, Nankai University, Tianjin, China, and, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, and colleagues have developed a nickel(II)-catalyzed lactamization of aryl C–H bonds in 2-arylanilines with CO2 to obtain phenanthridinones (general product structure pictured). The team used NiCl2·DME (DME = dimethoxyethane) as the catalyst together with Sphos (2-dicyclohexylphosphino-2′,6′-dimethoxybiphenyl) as a ligand, tBuONa as a base, and LiCl as an additive. The reactions were performed in dimethylformamide (DMF) at 110 °C under 1 atm of CO2.
Under these conditions, the desired phenanthridinones were obtained in moderate to excellent yields. The team proposes a reaction mechanism that involves the reaction of the 2-arylaniline with the nickel complex under deprotonation to form an intermediate complex. This complex is converted to a nickelacycle via C–H bond activation, followed by CO2 insertion and formation of the lactam.
- Nickel-Catalyzed Lactamization Reaction of 2-Arylanilines with CO2,
Lu Wang, Hanxuan Wu, Yucheng Zhao, Bin Li, Baiquan Wang,
Org. Lett. 2024.
https://doi.org/10.1021/acs.orglett.4c01156