Conventional cooling systems, such as air conditioners, work by causing a refrigerant to cycle between being a gas or a liquid. When the liquid becomes a gas, it expands and absorbs heat. This effect can be used to cool a room or the inside of a refrigerator. A compressor turns the gas back into a liquid and releases the heat. While this cycle is efficient, concerns about climate change and stricter regulations on fluorinated hydrocarbons are driving the search for more environmentally friendly refrigerants.
Refrigerants that undergo phase transitions in the solid state could be a solution. In such barocaloric materials, the temperature at which it transitions from a low-entropy (more ordered) to a high-entropy (more disordered) state changes dramatically as pressure is applied. However, most of these materials require tremendous pressure to drive the phase transition. This requires expensive specialized equipment that is impractical for real-world cooling applications.
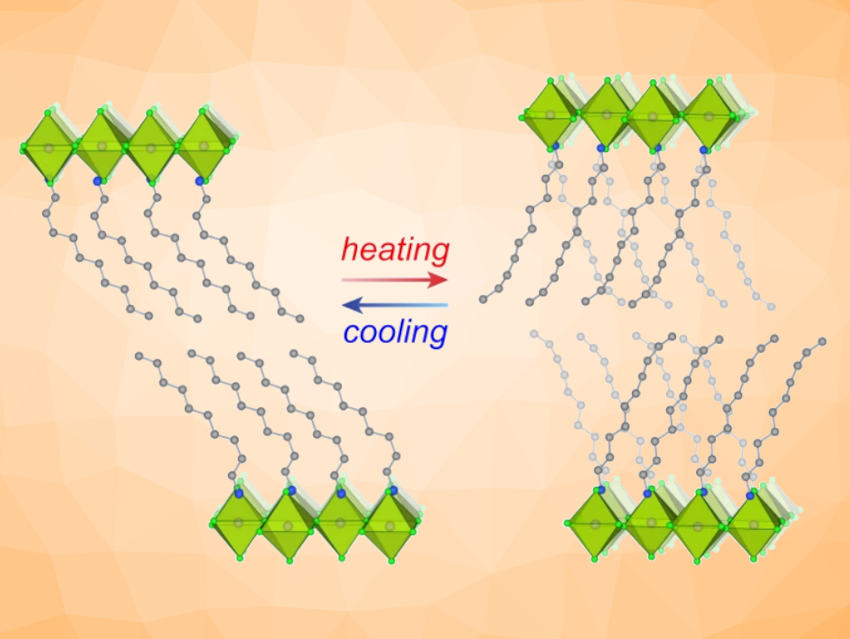
Jarad A. Mason, Harvard University, Cambridge, MA, USA, and colleagues have built the first prototype demonstrating the use of barocaloric materials as functional refrigerants in a practical cooling system. They use two representative 2D perovskites, (DA)2MnCl4 (DA = decylammonium) and (NA)2CuBr4 (NA = nonylammonium), that can undergo reversible phase transitions with large entropy changes, high sensitivity to pressure, and minimal hysteresis.
Their device consists of three main parts: (i) a metal tube filled with the solid refrigerant and an inert liquid such as water or oil, (ii) a hydraulic piston that applies pressure to the liquid, and (iii) the liquid that transfers the pressure to the refrigerant and transports the heat through the system.
The long, flexible molecular chains of the metal–halide perovskites (pictured above) become more ordered and rigid under pressure, which releases heat. The system does not yet operate at pressures as low as commercial refrigeration systems. However, the researchers believe that it is possible to achieve phase transitions with even higher entropy changes by functionalizing the organic bilayers, e.g., by introducing hydrogen bond donor-acceptor pairs. With optimization, solid-state refrigerants could become viable replacements for current air conditioning and other cooling technologies.
- Colossal barocaloric effects with ultralow hysteresis in two-dimensional metal–halide perovskites,
Jinyoung Seo, Ryan D. McGillicuddy, Adam H. Slavney, Selena Zhang, Rahil Ukani, Andrey A. Yakovenko, Shao-Liang Zheng, Jarad A. Mason,
Nat. Commun. 2022.
https://doi.org/10.1038/s41467-022-29800-9
The research was presented at the fall meeting of the American Chemical Society (ACS Fall 2022) held from August 21–25, 2022.
Also of Interest
Interview: Magnetocaloric Refrigeration: Reinventing the Fridge?
ChemistryViews 2015.
Professor Ekkes Brück, Delft University, about magnetocaloric cooling